HealthManagement, Volume 24 - Issue 1, 2024
An overview of the Procure4Health working group for precision medicine and the key areas of focus where innovation could alleviate challenges associated with its implementation.
Key Points
- Procurement of innovation is about improving public services by adopting innovative solutions to tackle the challenges faced by public services.
- The Procure4Health approach involves embracing innovative solutions to address the challenges faced by healthcare organisations.
- The Procure4Health community focuses on building capacities within healthcare systems regarding PI, facilitating collaboration among procurers of innovation in the health sector and shaping policies to influence the future of the PI instrument.
- The Procure4Health working group for precision medicine uncovered seven areas of focus where innovation could alleviate challenges, and three topics related to the market analysis phase.
Procurement of innovation is about improving public services by adopting innovative solutions to tackle the challenges faced by public services. Procure4Health is a community tasked with enhancing healthcare organisations including hospitals, regional and national public health systems, private health systems, and central purchasing bodies.
The Procure4Health approach involves embracing innovative solutions to address the challenges faced by healthcare organisations. Adopting innovations translates to procurement of innovations (PI) for healthcare systems. Recognising the potential of the PI instrument to drive significant change in healthcare systems across Europe, the European Commission has entrusted the Procure4Health community with the mission to promote its adoption. To fulfil this mandate, the Procure4Health community focuses on building capacities within healthcare systems regarding PI, facilitating collaboration among procurers of innovation in the health sector and shaping policies to influence the future of the PI instrument.
Procuring Innovation: A Collaborative Approach to Co-Creating Healthcare Solutions
In today's landscape, enhancing healthcare and care services requires embracing innovations that address existing challenges. This entails procuring innovations tailored to specific needs for healthcare systems rather than settling for off-the-shelf solutions that may only partially meet requirements. The procurement of innovation instrument begins by defining needs and engaging with the market/innovation ecosystem, inviting them to develop solutions. Moreover, potential developers are incentivised by not only the procurement of the outcome but also by being supported in the later stages of development. This collaborative approach underscores the paradigm of co-creation, a journey requiring joint efforts from both the demand and supply sides to achieve success.
Procurement Innovation - Concrete Impact on Healthcare
Procurement innovation is all about meeting the needs of end-users, clinicians, and patients, ultimately enhancing their experiences. Successful procurement innovation results in concrete solutions for diagnosis, treatment, or procedures that improve the daily lives of clinicians and, consequently, patients. Key steps for adopting the PI instrument within an organisation include:
- Allocating human resources to oversee the process, establishing a technical office for procurement of innovation.
- Defining organisational strategy and priorities.
- Engaging internally with end-users to identify unmet needs for innovation.
- Prioritising these unmet needs.
- Sharing this information with the market/innovation ecosystem and soliciting feedback on potential solutions.
- Initiating the tender process to contract solution development.
- Evaluating proposed solutions.
- Scaling up and deploying successful solutions.
However, success hinges on obtaining high-level management endorsement for the technical office to procure innovation. Procure4Health adopts a practical, hands-on approach rooted in the real experiences and challenges of our community members. We address requirements, obstacles, and approaches to innovative solutions, culminating in joint recommendations for top management and policymakers of healthcare systems.
Participation in Procurement Innovation
The profiles of the active members of the P4H community are as follows: management, procurement, innovation and ICT departments. Organisation-wise, the core audience is the procurers in the healthcare sector. But, as the procurement of innovation journey is the paradigm of co-creation, all the stakeholders have an important role to play at various points of the journey. Thus, P4H is open to everyone: procurers of innovations, supporting organisations, and suppliers. However, depending on the nature of each activity and to improve the efficiency of a specific discussion, each activity might be directed to a more precise group of stakeholders.

Twinnings and Clinical Working Groups: Structure for Uptake of Innovation
P4H has two main ways to structure the work and enable joint discussions between stakeholders:
- Twinnings. Partnerships among procurers to share best practices, expertise and strategy.
- Working groups (WG). There are four clinical working groups dedicated to agreeing on common unmet needs of innovation, performing the market analysis of those needs, exploring the requirements for the development, scaling and deployment of the innovative solution in our organisations and delivering recommendations for top health management and policy maker to foster the uptake of the innovative solution developed. These working groups are:
- Precision and Predictive Medicine
- Digital Health and ICTs
- Sustainability in Procurement of Innovation
- Integrated Care
Two working groups focus on how to improve the efficiency of the PI instrument:
- Value-based healthcare WG tackles how to incorporate value base criteria in the tender process to acquire the development of innovative solutions.
- Impactful innovation WG studies the impact and how to overcome the final "valley of death" of the innovation process. How to go from a successfully piloted solution to deployment in our healthcare systems.
Precision Medicine Challenges and Unmet Needs: A Call for Innovation
Precision medicine represents a paradigm shift in healthcare, aiming to customise treatment strategies according to individual patient characteristics, encompassing genetics, environment, and lifestyle factors.
At the heart of precision medicine lies the concept of genomic profiling. Genomic profiling entails the comprehensive examination of an individual's genetic composition, including variations in their DNA sequence. Recent advancements in high-throughput DNA sequencing technologies have made obtaining a person's complete genomic profile increasingly feasible and affordable. These profiles provide valuable insights into an individual's predisposition to certain diseases, their treatment response, and their susceptibility to adverse drug reactions.
The future of medicine lies in providing the right treatment for the right patient at the right time, emphasising personalised interventions based on biomarker responses. Within the broader scope of personalised healthcare, precision medicine integrates genomic, proteomic, and lifestyle data to create tailored prognostic, diagnostic, and therapeutic interventions, revolutionising the landscape of modern medicine.
The Procure4Health working group for Precision Medicine uncovered seven areas of focus where innovation could alleviate challenges, and three topics entered the market analysis phase to be selected to enter the next phase of the procurement of innovation journey, the Open Market Consultation (OMC).
- Genomic neonatal screening: This need is based on what is known as "diagnostic opportunity" and would be framed within a population screening programme for secondary prevention. The main problem detected in the public domain is that many patients affected by rare diseases are diagnosed with an average delay of 4 years. The reasons for this diagnostic delay are diverse: little knowledge of the disease, non-specific symptoms, few specialists trained in this field, poor access to highly complex genomic tests, etc. This means that, in many cases, when patients are diagnosed, they are no longer candidates for treatment because they are at a too-advanced stage of the disease. These patients often present sequelae or irreversible deterioration, manifesting incapacity, dependence, hospitalisation or frequent medical visits, entailing a high cost to the health system and society. Detecting all treatable rare diseases at birth through genetic newborn screening would significantly accelerate and improve the percentage of patients diagnosed (on time). Patients could then access treatment in the pre-or paucisymptomatic phase, improving their survival, morbidity and quality of life. It would also bring considerable savings to the healthcare system (less or less expensive treatments, fewer hospitalisations, fewer resources in general, etc.). There is currently no market solution to address this need. It would be necessary for the genetic panel adapted to DNA extraction in dried blood to be flexible and updatable (genes can be easily added) and for the response time for results to be less than ten days.
- Preservation of fresh biological samples: New molecular techniques in research (and increasingly in clinical practice) require the availability of biological material from patients preserved in a system that does not modify the nucleic acids, proteins or metabolites of the sample and that does not require freezing and the associated infrastructure. Frozen tissue is the best biological sample for current molecular biology techniques, but the costs of frozen storage are very high. The widespread method of storing patient samples is by fixation and embedding in paraffin, but this system does not allow us to get the most out of patient samples. Therefore, there is a need to develop other techniques to keep the sample as close as possible to the original state (at low costs, preferably).
- Improved monitoring and optimisation of resources for personalised lung cancer care: Nowadays, lung cancer is the paradigm of personalised medicine. Only 15-20% of cases are diagnosed at a limited stage, and surgery can be an active part of patient treatment. Therefore, speaking of curability, there has been a real diagnostic and therapeutic revolution in patients diagnosed at a locally advanced and/or metastatic stage in the last ten years. On the one hand, the possibility of genomic sequencing of patients, especially with non-small cell lung carcinoma (NSCLC) subtype adenocarcinoma, and their access to targeted therapy, as well as the development of immunotherapy, have increased patient survival in this setting from 5-10% to a 35-60% probability of being alive at five years. We can now speak of long survivors, with and/or without disease, with and/or without active treatment. Patient follow-up has never been protocolised other than consensus guidelines based on expert recommendations. The current challenge presented by this new scenario makes the need to standardise this follow-up a priority, which would have a clear impact on the continuity of patient care and the optimisation of resources. Therefore, the challenge here would be integrating data from patients' clinical histories with the results of their complementary tests (analytical, imaging tests, pathological anatomy-molecular biology studies, etc.) and developing predictive models based on the same.
Measuring Success: Impact, Widespread Implementation and Standardisation
Our measure of success aligns with our ability to make tangible improvements in the lives of end-users, clinicians, and patients. In our context, efficiency hinges on our capacity to effectively scale up and deploy successful pilots developed within our organisations.
While technological failures are inevitable in innovation activities, our focus remains on ensuring that solutions demonstrating technological success are seamlessly integrated and expanded across our healthcare settings. Efficiency means not only achieving technological milestones but translating those achievements into widespread implementation and meaningful impact on patient care and outcomes.
Standardisation streamlines processes and enhances efficiency. By establishing standardised procedures for assessing, defining, and prioritising innovation needs, we can ensure a systematic and proactive approach to innovation development and uptake. This shift from reactive to proactive engagement allows us to strategically allocate resources and maximise the impact of our initiatives.
Standardisation also fosters scalability and replicability. By standardising procurement processes and methodologies, we pave the way for the mass production of innovation projects. This transition from bespoke, handcrafted projects to standardised, replicable models holds the potential to significantly enhance the efficiency and effectiveness of innovation procurement.
Ultimately, by embracing standardisation as a core principle, P4H aims to revolutionise the landscape of healthcare innovation, driving widespread adoption and amplifying impact on a global scale.
High-Level Endorsement to Scaling Up and Reach Critical Mass
The strategy to scale up and achieve the critical mass necessary for global impact revolves around a comprehensive action plan developed within Procure4Health (P4H). This action plan encapsulates the approach, requirements, and recommendations for effectively adopting innovations to enhance health care services.
The next pivotal objective for the Procure4Health community is to garner endorsement for this action plan, particularly from policymakers and top healthcare managers. Securing their support is essential to drive widespread adoption of our initiatives and ensure that our efforts are integrated into broader healthcare policies and strategies.
By gaining endorsement at the highest levels of governance within the healthcare sector, we aim to create an enabling environment conducive to scaling up our initiatives and reaching the critical mass needed to deliver meaningful global impact. This strategic approach underscores our commitment to driving positive change and transforming healthcare delivery on a global scale.
Best-in-class Methodology to Navigate Innovation Needs
The assessment and prioritisation of innovation needs is the first step of the procurement of innovation journey. A combination of two methodologies is used for this process:
- EAFIP methodology https://eafip.eu/
Developed by one of our core partners CORVERS https://corvers.com/ - The Early Detection Map methodology developed by the coordinators of Procure4Health, the Andalusian Public Health System (APHS)
The main aim of the Early Demand Map is to obtain a portfolio of needs and opportunities for innovation that will transform the future Health Systems.
The initiative is based on co-creation models that stand for open innovation and design thinking methods to collect ideas and innovation needs. In this process, two levels of collaboration are very much promoted:
- Internal collaboration between professionals from the Andalusian Public Health System: researchers, clinicians, project technicians, managers, top managers and professionals with different expertise.
- External collaboration between professionals from external organisations who actively collaborate with the APHS professionals to contrast and define the needs identified and to offer guidance on possible solutions to the proposed challenges:
- Public sector (professionals from the Andalusian Public Health System).
- Private sector (companies, SMEs and startups).
- Academic sector (research centres, universities).
- Patient´s associations.
This collaboration happens along the different phases of the process, as is shown in the graphic below:
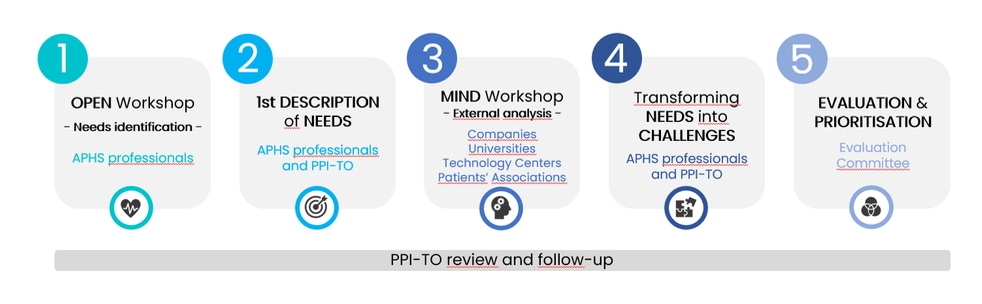
Professionals from all these sectors gather in the OPEN and MIND workshops to define the innovation needs of the Andalusian Public Health System. With the support of the Technical office, the needs are turned into challenges which, later on, will be implemented as public procurement of innovation projects which, ultimately, will contribute to the modernisation of the public system.
Communication and Advocacy Efforts to Raise Awareness
Engaging with high-level healthcare stakeholders to secure endorsement for our initiatives presents certain challenges, primarily due to the multifaceted nature of our approach and the need for collaboration across diverse departments and stages of the innovation journey.
The precision medicine ecosystem, encompassing patients, clinicians, researchers, technologies, genomics, and data sharing, requires a concerted effort from various stakeholders. Integration of precision medicine with innovation procurement, ICTs, legal considerations, and technology transfer further adds complexity to the engagement process.
Securing high-level endorsement is crucial to mobilise this diverse ecosystem and ensure collaborative action. While top management typically prioritises short-term objectives, our strategy represents a mid to long-term approach with inherent uncertainties. However, with clear support from the European Commission and numerous national governments for implementing the procurement of innovation instruments, there is a compelling rationale for swift adoption. Delaying adoption risks missing out on valuable opportunities to leverage public resources for fostering innovation and enhancing public services.
Raising awareness about the importance of our project is paramount to its success. By highlighting the potential benefits of precision medicine and innovation procurement, we aim to garner support and foster collaboration among stakeholders. Emphasising the alignment of our objectives with broader healthcare priorities and policy initiatives underscores the relevance and urgency of our endeavours. Through strategic communication and advocacy efforts, we seek to drive awareness and build momentum for transformative change in healthcare delivery.

Procure4Health is a platform for exchanging knowledge, sharing best practices, and fostering collaboration among healthcare stakeholders. Together, we can overcome challenges and drive positive change in healthcare delivery. Join us in shaping the future of healthcare innovation.
Conflict of Interest
None.
















