According to the National Cancer Institute, the incidence of cervical cancer in the United States has been steadily declining in recent decades, from an estimated 11.2 cases per 100,000 women in 1992 to 6.7 cases per 100,000 women in 2019. Statistics from other parts of the world, however, paint a substantially different picture.
In the United States, the incidence in 2019 is nearing what the World Health Organisation (WHO) considers the threshold of near-elimination, defined as 4 cases per 100,000 women. In fact, a 2021 study published in CancerEpidemiology, Biomarkers & Prevention, a journal of the AACR, showed that some areas of the United States may reach near-elimination by 2030.
Meanwhile, cervical cancer ranked as the fourth most common cancer worldwide in 2020, causing an estimated 342,000 deaths that year.
Around 90% of those deaths occurred in low- and middle-income countries (LMICs).
In 2020, the WHO launched the Cervical Cancer Elimination Initiative, outlining goals that, if met by 2030, could spur global near-elimination of cervical cancer by 2100. The goals include:
- vaccinating 90% of girls by age 15;
- screening 70% of women for HPV infection by age 35 and again by age 45; and
- delivering adequate treatment to 90% of cervical cancer patients.
These goals rely on trained providers and resources that may be scarce in LMICs. Fortunately, creative solutions engineered by researchers around the world using resource-conscious ways to vaccinate, screen, and treat patients with cervical cancer provide hope that these goals may be attainable.
Facilitating Self-Administered Treatment
Chemtai Mungo, MD, MPH, an assistant professor of obstetrics and gynecology at the University of North Carolina Lineberger Comprehensive Cancer Center, has seen firsthand the challenges that LMICs face when battling cervical cancer. Having grown up in Kenya and undergone medical training in the United States, she is quite familiar with the differences in cervical cancer management between the two countries.
“During my training, I almost never took care of a woman with cervical cancer; I never saw women dying from cervical cancer in the United States,” she said in an interview with AACR Stories. “But when I did spend time abroad, there were a lot of women with advanced cases dying.”
 Mungo was honored as a NextGen Star at the AACR Annual Meeting 2023, where she explained some of the reasons underlying the stark disparities.
Mungo was honored as a NextGen Star at the AACR Annual Meeting 2023, where she explained some of the reasons underlying the stark disparities.
The low prevalence of obstetrician/gynecologists in Kenya can make it difficult to secure an appointment, and patients sometimes have to travel significant distances to see a specialist.
Further, infection with the human immunodeficiency virus (HIV) can weaken women’s immune systems, making it harder to fight off the HPV infection that causes most cases of cervical cancer.
An estimated 67% of people living with HIV worldwide reside in sub-Saharan Africa.
Mungo hypothesized that a cheaper, more accessible treatment method may help reach women with high-risk cervical lesions or HPV infections that won’t go away on their own. She and her colleagues are currently testing the feasibility of a self-administered intravaginal chemotherapy treatment for such patients.
While studies performed in the United States have demonstrated preliminary efficacy of the method — both for the first-line treatment of patients who could not undergo surgery and to prevent relapse in patients post-surgery — Mungo sought to explore whether the solution might be practical in Kenya.
Mungo and colleagues have two ongoing studies exploring these issues. One—funded in part by the Victoria’s Secret Global Fund for Women’s Cancers Career Development Award, in partnership with Pelotonia and the AACR—aims to study treatment uptake, adherence to the treatment schedule, and treatment safety in women who are offered self-administered therapy after a surgical excision or ablation procedure. In the other, researchers are performing qualitative interviews with women who receive the self-administered treatment to gain a better understanding of their experience, including their safety, privacy, and ability to negotiate sexual abstinence with their partners during treatment.
“Cancer is a disease that affects all people, not just those in the United States,” Mungo said. “Equity must not be focused on American cancer patients only, but globally, because innovation internationally will improve the lives of American patients and vice versa.”
Improving Screening
Effectively treating patients with cervical cancer or precancer requires the detection of cervical abnormalities, which often do not cause symptoms.
The WHO recommends regular cervical inspection and sampling of cervical tissue, but they also recommend at least two lifetime tests for HPV.
Studies have shown that self-collection of cells to test for HPV may be as effective as sampling performed by a health care provider.
This requires patients to visit a doctor’s office, which can create hurdles to treatment compliance, especially in LMICs.
 Numerous studies have evaluated vaginal self-sampling to detect HPV and found the efficacy to be similar to that of cervical sampling performed in a clinic.
Numerous studies have evaluated vaginal self-sampling to detect HPV and found the efficacy to be similar to that of cervical sampling performed in a clinic.
But questions remain regarding how well these tests identified patients with cervical lesions as well as what types of tests worked best.
A recent prospective study in Cancer Prevention Research, a journal of the AACR, evaluated the detection rates of high-risk (likely to cause cancer) HPV types in vaginal and cervical samples from the same 5,856 patients.
The study confirmed that the identification of high-risk HPV was similar between vaginal and cervical testing; 13.7% of cervical samples and 15.3% of vaginal samples tested positive.
Among those who tested positive for high-risk HPV, patients who were found to have CIN3—the highest grade of cervical precancer—had received similar results from both sampling modalities, with only one CIN3 case having different vaginal and cervical testing results.
Since follow-up clinical visits may pose challenges in resource-limited settings, the researchers also evaluated whether they could effectively triage patients who tested positive for high-risk HPV to determine who should be referred for intensive cervical examination. Among self-collected vaginal samples, detection of the two most carcinogenic HPV types—HPV16 and HPV18—appeared to identify precancerous lesions with the best sensitivity and specificity. Testing for the top five HPV types resulted in a similar sensitivity but with a much higher false-positive rate.
Therefore, the researchers concluded that vaginal self-sampling followed by genotyping for HPV16 and HPV18 may efficiently and accurately identify patients with precancerous lesions who may require treatment and follow-up. In low-resource settings, these measures may reduce the costs and logistical hurdles associated with effective screening.
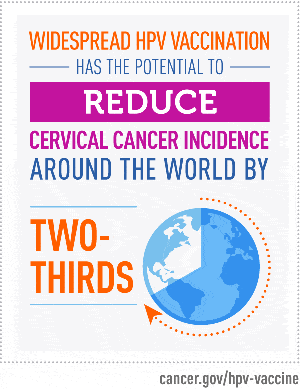 Expanding Vaccine Access
Expanding Vaccine Access
Graphic courtesy of the National Cancer Institute.
Because HPV causes more than 90% of cervical cancer cases, reducing the spread of HPV can potentially prevent almost all cases of cervical cancer. In the United States, vaccines against HPV first became available in 2006 and are recommended for all adolescents before the onset of sexual activity.
The U.S. Centers for Disease Control and Prevention (CDC) recommend a two-dose series for children who receive the vaccine between ages 9 and 15 and a three-dose series for individuals aged 15 to 26. The vaccine is offered to both boys and girls because vaccinating boys can help decrease the spread of HPV, and because HPV increases the risk of certain cancers in men, such as anal, penile, and oropharyngeal cancers.
Guidelines and rates of vaccine uptake may vary, however, in settings where vaccine doses are harder to come by and multiple vaccination appointments may be unrealistic. For that reason, the WHO had long recommended a two-dose series, predominantly for girls.
But what if one dose can provide similar protection to two? Could that eliminate some of the resource strain and help vaccinate more children?
A handful of recent trials have tested this approach. In the KEN SHE trial, researchers vaccinated Kenyan girls and women aged 15 to 20 with a single dose of either a bivalent vaccine (targeting HPV16 and HPV18) or a nonavalent vaccine (targeting nine HPV types, including seven associated with cervical cancer).
At 18 months post-vaccination, both vaccines were 97.5% effective at preventing persistent HPV infections. The researchers intend to follow up on these patients at regular intervals to monitor the continued efficacy of the vaccine.
Similar findings have been reported in trials monitoring patients for 10 years after receiving a single bivalent vaccine dose in Costa Rica or a single quadrivalent (targeting four HPV types) vaccine dose in India.
Based on these and other studies, in December 2022, the WHO updated their HPV vaccination guidelines to allow for the use of a single vaccine dose for girls aged 9 to 20; individuals older than 20, who have HIV, or who are immunocompromised should still receive at least two doses.
Officials hope the new guidelines will improve vaccine access by freeing up more doses and eliminating logistical challenges for patients needing to return for a second dose.
“Averting the development of cervical cancer by increasing access to effective vaccines is a highly significant step in alleviating unnecessary illness and death,” a WHO spokesperson wrote in a news release about the update.
Source: AACR