ICU Management & Practice, Volume 24 - Issue 1, 2024
Corticosteroids are commonly used drugs in multiple diseases and conditions of critically ill patients. In this article, we review the pharmacology of corticosteroids and provide recommendations for their use in the ICU based on the best available evidence.
Corticosteroids: Pharmacology and General Aspects
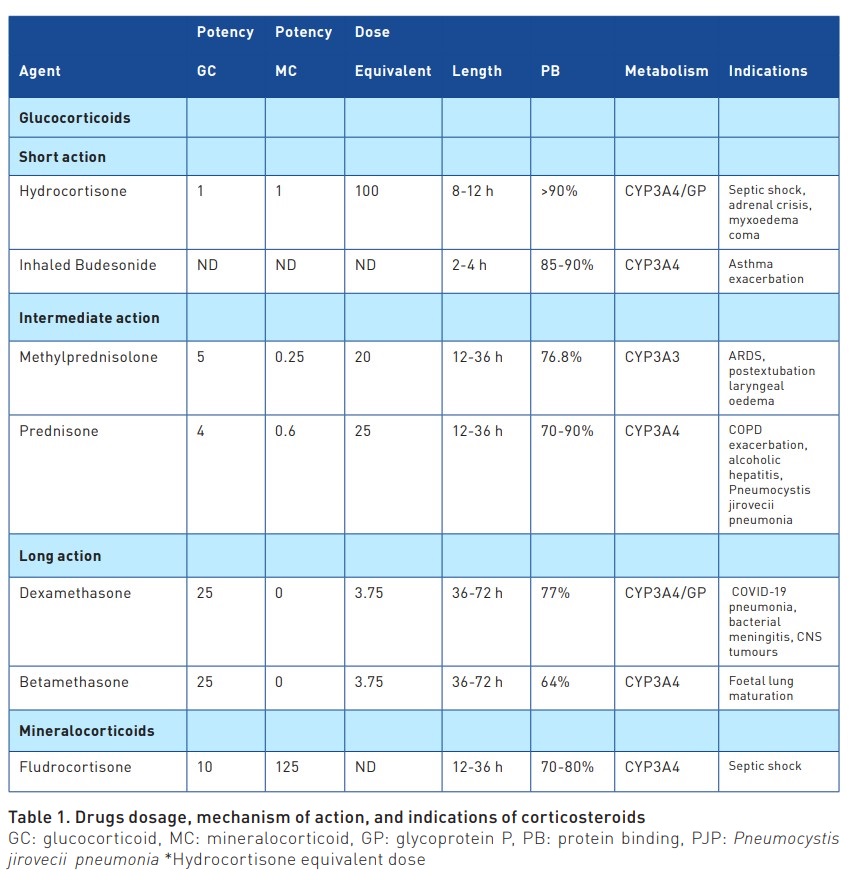
Corticosteroids are hormones synthesised in the adrenal cortex from cholesterol. They are classified into mineralocorticoids and glucocorticoids. Their functions include maintaining water and electrolyte balance, anti-inflammatory effects, regulation of blood pressure, immunosuppression, glycaemic control, and other metabolic pathways (Young and Marsh 2018). Exogenous or synthetic corticosteroids exhibit glucocorticoid and mineralocorticoid properties to varying degrees (Table 1). Most circulating steroids bind to plasma proteins such as corticosteroid-binding globulin (CBG), albumin, and alpha-1 acid glycoprotein. In critically ill patients (e.g., sepsis, severe burns, or acute myocardial infarction), CBG rapidly decreases its plasma concentration, increasing the amount of free glucocorticoids that can control the inflammatory response, gluconeogenesis, and stress (Gardill et al. 2012). The effect of cortisol may be insufficient due to adrenal dysfunction and corticosteroid resistance to counteract an exaggerated and prolonged inflammatory response (Keh 2016). Exogenous corticosteroids enter cells by binding to the glucocorticoid receptor, exerting their action in the nucleus, where they bind to DNA, generating down-regulation of the release of inflammatory substances (Barnes 2011). Side effects at moderate or high doses may be associated with an increased infection rate, longer ICU stay, more ventilator days, and a tendency towards higher mortality (Britt et al. 2006), as well as myopathies, gastrointestinal bleeding, fluid retention, and psychotic reactions (Yasir et al. 2023).
In this article, we review the pharmacology of corticosteroids and provide recommendations for their use in the ICU based on the best available evidence.
Septic Shock
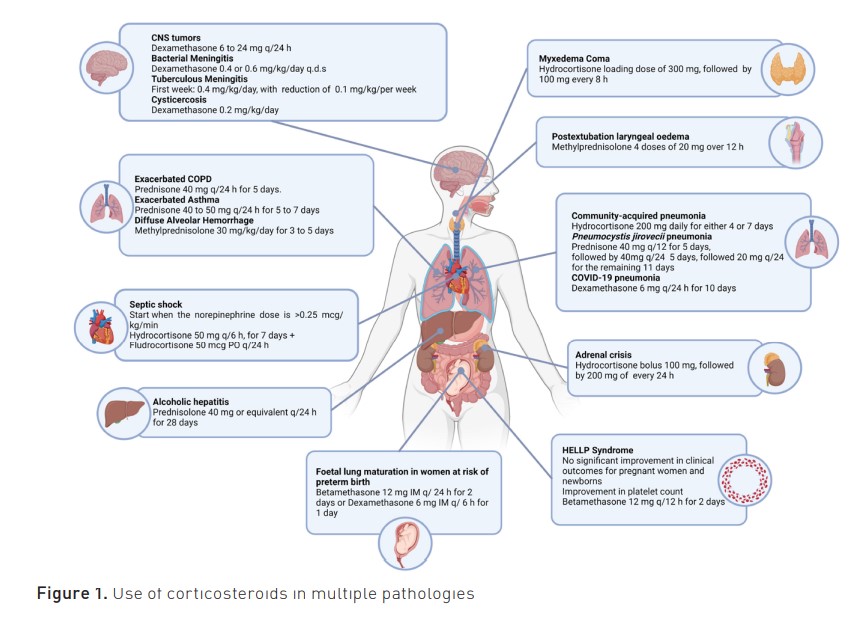
Corticosteroids have been under evaluation as adjunctive therapy for septic shock for over 50 years. In the latest meta-analysis, the relative risk (RR) for 90-day mortality in patients with septic shock, comparing hydrocortisone to placebo, was 0.93 (95% CI, 0.82 to 1.04; p=0.22). This value was 0.86 (95% CI, 0.79 to 0.92) for the combination of hydrocortisone and fludrocortisone and 0.96 (95% CI, 0.82 to 1.12) without fludrocortisone (Pirrachio 2023). Itis recommended to initiate corticosteroids when a norepinephrine dose of 0.25 mcg/kg/min is reached.
Community-Acquired Pneumonia and Pneumocystis jirovecii Pneumonia
A randomised controlled study demonstrated a decrease in mortality among patients with severe community-acquired pneumonia (CAP), defined as the initiation of invasive mechanical ventilation (IMV) or non-invasive mechanical ventilation with at least 5 cm H2O of PEEP, administration of high-flow nasal cannulas, PaO2/FiO2 less than 300, and PORT PSI of at least 130 points. The study found a reduction in mortality in patients who were administered hydrocortisone compared to placebo (absolute difference, -5.6%, 95% CI, -9.6 to -1.7; p=0.006), a decrease in intubations (HR, 0.59; 95% CI, 0.40 to 0.86), and a reduction in the use of vasopressors (HR, 0.59; 95% CI, 0.43 to 0.82) (Dequin et al. 2023). A systematic review with meta-analysis demonstrated that the use of corticosteroids reduced the risk of all-cause mortality in CAP (RR: 0.69, 95% CI: 0.53-0.89), especially in younger patients (Cheema et al. 2024; Chaudhuri et al. 2024).
In patients with pneumocystis jirovecii pneumonia, a systematic review and meta-analysis showed a decrease in mortality associated with the administration of 40 mg of prednisone twice daily for five consecutive days, followed by 40 mg once daily for the next five days and 20 mg once daily for the remaining 11 days (21 days) (RR 0.59, 95% CI, 0.41 to 0.85) (Hannah et al. 2015).
COVID-19 Pneumonia
In the RECOVERY study on COVID-19, the administration of 6 mg of dexamethasone per day to patients receiving supplementary oxygen or respiratory support was associated with a reduction in 28-day mortality in those undergoing IMV (RR 0.64; 95% CI, 0.51 to 0.81) and those receiving oxygen therapy (RR 0.82; 95% CI, 0.72 to 0.94) (RECOVERY Collaborative Group 2021). A systematic review and meta-analysis on the use of corticosteroids in patients with COVID-19 pneumonia demonstrated a decrease in mortality with the use of corticosteroids (OR 0.72, 95% CI 0.57-0.87). Viral clearance, superinfections, and the use of antibiotics were more common in this cohort (van Paassen et al. 2020).
Acute Respiratory Distress Syndrome
The use of corticosteroids in patients with acute respiratory distress syndrome (ARDS) is reserved for the following causes: CAP, COVID-19, pneumocystis jirovecii and diffuse alveolar haemorrhage (lack of evidence). Initiating corticosteroids after 14 days of IMV is not recommended, and adverse effects should be monitored, including immunosuppression, bacterial, fungal, parasitic, or mycobacterial infections (Qadir et al. 2024).
Postextubation Laryngeal Oedema
In patients who have failed the cuff leak test but are otherwise deemed ready for extubation based on other assessments, it is recommended to administer corticosteroid therapy at least four hours before extubation. It is suggested to give four doses of 20 mg of methylprednisolone over 12 hours, which reduces the incidence of postextubation laryngeal oedema (3% vs 22%, p < 0.0001), the overall incidence of reintubations (4% vs 8%, p = 0.02), and the proportion of reintubations secondary to laryngeal oedema (8% vs 54%, p = 0.005) (Francois et al. 2007).
Exacerbated COPD
According to the GOLD 2024 guidelines, the use of systemic corticosteroids is recommended during COPD exacerbations, with a maximum duration of five days and a daily dose of 40 mg of prednisone (or equivalent). This treatment approach is found to be equally effective through enteral or parenteral administration (GOLD 2024). Corticosteroids have been shown to reduce recurrence rates and the need for hospitalisation in patients experiencing COPD exacerbations. There is a significant improvement in lung function test results and a decrease in the risk of treatment failure in both outpatient and hospitalised patients when compared to placebo (OR 0.48; 95% CI 0.35, 0.67), without a significant difference in mortality (Walters et al. 2014).
Exacerbated Asthma
It is recommended to use inhaled corticosteroids for the treatment of exacerbated asthma. The benefits of this approach include acute relief of symptoms, a reduction in the risk of severe asthma attacks, a lower likelihood of hospitalisation, and the avoidance of initiating oral corticosteroids (Global Initiative for Asthma 2023). Oral corticosteroids are recommended for patients who, despite using inhaled corticosteroids for 2-3 days, fail to improve their symptoms during an asthma attack. The recommended dose is 40-50 mg/day of prednisone for 5 to 7 days (Normansell et al. 2016). In patients presenting to the emergency department with asthma exacerbation, the administration of corticosteroids via any route (IV, oral, IM, or inhaled) is recommended within the first hour of admission. This measure has been shown to reduce the probability of requiring hospitalisation by 50% (0.50 95% CI 0.31-0.81 p=0.004) (Rowe et al. 2001).
Adrenal Crisis
Adrenal crisis or insufficiency is defined as an acute deterioration in health associated with absolute hypotension (systolic blood pressure <100 mmHg), with characteristics resolving after the parenteral administration of glucocorticoids (demonstrated by a marked resolution of hypotension within one hour and improvement of clinical symptoms within two hours) (Rushworth et al. 2019). There are no controlled clinical trials regarding the treatment. The use of parenteral hydrocortisone is suggested. It is administered through a bolus injection of 100 mg IV or IM (while awaiting IV access). This bolus should be followed by 200 mg of hydrocortisone every 24 hours, either through continuous IV infusion or, alternatively, in doses of 50 mg of hydrocortisone per IV/IM injection every six hours (Arlt 2016).
Bacterial Meningitis
A systematic review and meta-analysis of randomised controlled trials on bacterial meningitis found that patients administered dexamethasone versus placebo had significantly lower rates of severe hearing loss (RR 0.67; 95% CI: 0.51 to 0.88), a decrease in the incidence of any hearing loss (RR 0.74; 95% CI), and neurological sequelae (RR 0.83; 95% CI: 0.69 to 1.00; p=0.05). An analysis focused on different bacteria causing meningitis demonstrated that patients with Streptococcus pneumoniae meningitis treated with corticosteroids had a lower mortality rate (RR 0.84; 95% CI: 0.72 to 0.98). Most studies utilised a four-day regimen of dexamethasone (0.4 or 0.6 mg/kg/day) administered in four daily doses (Brouwer et al. 2015).
Tuberculous Meningitis
A systematic review and meta-analysis of randomised controlled trials on tuberculous meningitis demonstrated that corticosteroids reduce the risk of death by 25% between two months and two years after initiating treatment (RR 0.75; 95% CI 0.65 to 0.87). In most studies, a dexamethasone dose reduction regimen was employed over the course of one month, distributed as follows: in week 1, 0.4 mg/kg/day for 7 days; in week 2, 0.3 mg/kg/day for 7 days; in week 3, 0.3 mg/kg/day for 7 days; and in week 4, 0.1 mg/kg/day for 7 days (Prasad et al. 2016).
Cysticercosis
In cases of viable intraparenchymal neurocysticercosis with multiple enhanced lesions, the use of anti-inflammatory therapy with corticosteroids is suggested to manage diffuse cerebral oedema. This therapy should be administered before initiating treatment with antiparasitic drugs, with a strong-moderate recommendation. In the case of subarachnoid neurocysticercosis, chronic use of high-dose steroids is advised, in addition to requiring intensive antiparasitic therapy and, in some cases, surgical intervention. Although the doses are not fully standardised, the administration of dexamethasone at 0.2 mg/kg/day may be considered, according to the strong-moderate recommendation. For patients with spinal neurocysticercosis and spinal cord dysfunction, such as paraparesis or incontinence, the use of corticosteroids is suggested as part of adjunctive management along with antiparasitic therapy, with a strong-moderate recommendation (White et al. 2017).
Myxoedema Coma and Thyroid Storm
The treatment of myxoedema coma primarily revolves around the use of thyroid hormones (Ylli et al. 2019). The administration of corticosteroids is recommended as part of the initial treatment for myxoedema coma, involving intravenous administration at appropriate stress doses before levothyroxine administration (Jonklaas et al. 2014). It is essential to note that this recommendation lacks support from controlled clinical trials. In cases of thyroid storm, a loading dose of 300 mg of hydrocortisone is suggested, followed by 100 mg every 8 hours (ATA guidelines 2016). However, it is important to acknowledge that the supportive evidence for this recommendation is not derived from large-scale clinical trials (Ross et al. 2016). A nationwide retrospective study indicated that corticosteroids do not improve survival in patients with thyroid storm. Moreover, they also induce glycaemic dysregulation and an increased need for insulin (Senda et al. 2020).
Alcoholic Hepatitis
A systematic review and meta-analysis revealed that corticosteroid monotherapy (prednisolone 40 mg or equivalent for 28 days) significantly reduced mortality compared to placebo (OR=0.58; 95% CI, 0.34-0.98; P=0.04) (Sung-Lee 2017).
Central Nervous System Tumours
Dexamethasone has been used for malignant brain tumours. It is employed to manage peritumoral oedema and alleviate symptoms related to intracranial hypertension. For the treatment of peritumoral oedema, described doses include 4 mg every 6 hours for 8 to 19 days, resulting in an average reduction in oedema volume (26%). Another regimen involves 4 mg every 6 hours for 7 days, followed by a maintenance dose of 4 mg/day until surgical intervention or radiotherapy, leading to an average reduction in oedema volume (56%). The following regimen is proposed based on symptom relief (Ly et al. 2017): initial one-time dose of 10 mg, followed by 4 mg every 6 hours
Diffuse Alveolar Haemorrhage
Diffuse alveolar haemorrhage (DAH) is a severe pulmonary complication that can occur in certain autoimmune diseases such as systemic lupus erythematosus, vasculitis associated with antineutrophil cytoplasmic antibodies (ANCA), antiphospholipid syndrome, and anti-glomerular basement membrane syndrome (Al-Adhoubi and Bystrom 2020). Corticosteroids are recommended in up to 98% of all cases. Their primary goal is to reduce the acute inflammatory response of the alveolar endothelium (Ednalino et al. 2015); however, to date, therapeutic recommendations for corticosteroids are based on retrospective trials and anecdotal reports. Despite the use of high-dose corticosteroids, mortality exceeds 50% (Park 2021).
HELLP Syndrome
A systematic review with meta-analysis (Sun et al. 2023) found no significant improvement in clinical outcomes for pregnant women and newborns with HELLP syndrome. However, the platelet count showed improvement after the administration of betamethasone at a dosage of 12 mg every 24 hours for two days (81.2%, 95% CI [43%, 533%]) compared to a placebo group (94.5%, 95% CI [24%, 627%]); there was no statistically significant difference between the two groups (p=0.23) (Ozer et al. 2009).
Foetal Lung Maturation in Women at Risk of Preterm Birth
Prenatal corticosteroid treatment in women at risk of preterm birth reduces the risk of respiratory distress (OR 0.66; 95% CI 0.54 to 0.82; p<0.001), mortality (OR 0.64; 95% CI 0.50 to 0.81; p<0.001), intraventricular haemorrhage, (OR 0.67; 95% CI 0.54 to 0.83; p<0.001), leukomalacia periventricular (OR 0.65; 95% CI 0.47 to 0.92; p<0.001) and necrotising enterocolitis compared to unexposed premature newborns. Corticosteroids should be initiated when a high risk of preterm birth is anticipated within the next one to seven days to achieve its effectiveness; if administered less than 24 hours before delivery, the effectiveness is incomplete. Betamethasone has shown a greater reduction in intraventricular haemorrhage. A course of therapy consists of intramuscular betamethasone 12 mg doses every 24 hours for two doses, or intramuscular dexamethasone 6 mg doses every 6 hours for four doses. An alternative is intravenous hydrocortisone 500 mg every 6 hours. (Dongkin et al. 2021).
Conclusion
The use of corticosteroids has demonstrated effectiveness in multiple pathologies in the ICU (Figure 1); however, clinical trials are needed to establish dosage and treatment duration for autoimmune and endocrine disorders.
Conflict of Interest
None.
References:
Al-Adhoubi NK, Bystrom J (2020) Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus. 29(4):355-363.
Arlt W (2016) Society for Endocrinology Clinical Committee. Society for Endocrinology Endocrine Emergency Guidance: Emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 5(5):G1-G3.
Arora H, Mammi M, Patel NM et al. (2023) Dexamethasone and overall survival and progression free survival in patients with newly diagnosed glioblastoma: a meta-analysis. J Neurooncol.
Barnes PJ (2011) Glucocorticosteroids: current and future directions. Br J Pharmacol. 163(1):29-43.
Britt RC, Devine A, Swallen KC et al. (2006) Corticosteroid Use in the Intensive Care Unit: At What Cost? Arch Surg. 141(2):145–149.
Brouwer MC, McIntyre P, Prasad K, van de Beek D (2015) Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. (9):CD004405.
Chaudhuri D, Nei AM, Rochwerg B et al. (2024) 2024 Focused Update: Guidelines on Use of Corticosteroids in Sepsis, Acute Respiratory Distress Syndrome, and Community-Acquired Pneumonia. Crit Care Med.
Cheema HA, Musheer A, Ejaz A et al. (2023) Efficacy and safety of corticosteroids for the treatment of community-acquired pneumonia: A systematic review and meta-analysis of randomized controlled trials. J Crit Care.
Cheng T, Gong Y, Guo Y et al. (2013) Systemic corticosteroid for COPD exacerbations, whether the higher dose is better? A meta-analysis of randomized controlled trials. Clin Respir J. 7(4):305-318.
Dequin PF, Meziani F, Quenot JP et al. (2023) Hydrocortisone in Severe Community-Acquired Pneumonia. N Engl J Med. 388(21):1931-1941.
Ednalino C, Yip J, Carsons SE (2015) Systematic Review of Diffuse Alveolar Hemorrhage in Systemic Lupus Erythematosus: Focus on Outcome and Therapy. J Clin Rheumatol. 21(6):305-310.
Ewald H, Raatz H, Boscacci R et al. (2015) Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst Rev. (4):CD006150.
François B, Bellissant E, Gissot V et al. (2007) 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. 369(9567):1083-1089.
García HH, Nash TE, Del Brutto OH (2014) Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 13(12):1202-1215.
Gardill BR, Vogl MR, Lin HY et al. (2012) Corticosteroid-binding globulin: structure-function implications from species differences. PLoS One. 7(12):e52759.
Global Initiative for Asthma (2023) Global Strategy for Asthma Management and Prevention, 2023. Updated July 2023. Available at www.ginasthma.org
Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Prevention, Diagnosis and Management of COPD: 2024 Report. Bethesda: GOLD. Available at https://goldcopd.org/2024-gold-report
Jessurun CAC, Hulsbergen AFC, Cho LD et al. (2019) Evidence-based dexamethasone dosing in malignant brain tumors: what do we really know? J Neurooncol. 144(2):249-264.
Jonklaas J, Bianco AC, Bauer AJ et al. (2014) Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 24(12):1670-1751.
Keh D (2016) Steroids in critical illness in Andrew Webb, and others (eds), Oxford Textbook of Critical Care, 2nd Edition Oxford Textbook.
Lee YS, Kim HJ, Kim JH et al. (2017) Treatment of Severe Alcoholic Hepatitis With Corticosteroid, Pentoxifylline, or Dual Therapy: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 51(4):364-377.
Lin D, Fan D, Chen G et al. (2021) Association of antenatal corticosteroids with morbidity and mortality among preterm multiple gestations: meta-analysis of observational studies. BMJ Open. 11(9):e047651.
Maspero JF, Cruz AA, Beltran CFP et al. (2023) The use of systemic corticosteroids in asthma management in Latin American countries. World Allergy Organ J. 16(4):100760.
Matchaba P, Moodley J (2004) Corticosteroids for HELLP syndrome in pregnancy. Cochrane Database Syst Rev. (1):CD002076.
McGoldrick E, Stewart F, Parker R, Dalziel SR (2020) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 12(12):CD004454.
Müller W, Kretzschmar K, Schicketanz KH (1984) CT analysis of brain tumors under steroid therapy. Neuroradiology. 26: 293–298.
Normansell R, Kew KM, Mansour G (2016) Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. (5):CD011801.
Ozer A, Kanat-Pektas M, Ozer S et al. (2009) The effects of betamethasone treatment on the clinical and laboratory characteristics of pregnant women with HELLP syndrome. Obstetricia Arch Gynecol. 280(1): 65–70.
Park JA (2021) Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int J Mol Sci. 22(2):793.
Pirracchio R, Annane D, Waschka A et al. (2023) Patient-Level Meta-Analysis of Low-Dose Hydrocortisone in Adults with Septic Shock. NEJM Evidence. 2.
Prasad K, Singh MB, Ryan H (2016) Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 4(4):CD002244.
Qadir N, Sahetya S, Munshi L et al. (2024) An Update on Management of Adult Patients with Acute Respiratory Distress Syndrome: An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 209(1):24-36.
RECOVERY Collaborative Group, Horby P, Lim WS et al. (2021)Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 384(8):693-704.
Roberts D, Brown J, Medley N, Dalziel SR (2017) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 3(3):CD004454.
Ross DS, Burch HB, Cooper DS et al. (2017) 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis [published correction appears in Thyroid. 27(11):1462. Thyroid. 26(10):1343-1421.
Rowe BH, Spooner C, Ducharme FM et al. (2001) Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. (1):CD002178.
Rushworth RL, Torpy DJ, Falhammar H (2019) Adrenal Crisis. N Engl J Med. 381(9):852-861.
Sarin R, Murthy V (2003) Medical decompressive therapy for primary and metastatic intracranial tumours. Lancet Neurol. 2(6):357-365.
Senda A, Endo A, Tachimori H et al. (2020) Early administration of glucocorticoid for thyroid storm: analysis of a national administrative database. Crit Care. 24(1):470.
Sun WJ, Hu J, Zhang Q, Shan JM (2023) Administration of corticosteroid therapy for HELLP syndrome in pregnant women: evidence from seven randomized controlled trials. Hypertens Pregnancy. 42(1):2276726.
Van Paassen J, Vos JS, Hoekstra EM et al. (2020) Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 24(1):696.
Walters JA, Tan DJ, White CJ, Wood-Baker R (2018) Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 3(3):CD006897.
Walters JAE, Tan DJ, White CJ et al. (2014) Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 9:CD001288.
White AC Jr, Coyle CM, Rajshekhar V et al. (2018) Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 66(8):e49-e75.
Yasir M, Goyal A, Sonthalia S (2023) Corticosteroid Adverse Effects. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Ylli D, Klubo-Gwiezdzinska J, Wartofsky L (2019) Thyroid emergencies [published correction appears in Pol Arch Intern Med. 129(9):653. Pol Arch Intern Med. 2019;129(7-8):526-534.
Young A, Marsh S (2018) Steroid use in critical care. BJA Educ. 18(5):129-134.




























