ICU Management & Practice, Volume 23 - Issue 4, 2023
Hospital and ICU nosocomial sepsis screening for acutely ill and high-risk patients and daily measurement of PSP to diagnose nosocomial sepsis three to five days before the onset of symptoms.
Introduction
Sepsis is a major public health threat and is responsible for 11 million deaths per year (Rudd et al. 2020) among 48.9 million cases. Sepsis and septic shock can be prevented if diagnosed and treated early by appropriate treatment, in particular, antibiotics. Mortality from sepsis increases by about 8% per hour of delayed appropriate administration of antibiotics (Kumar et al. 2006). Two campaigns at the worldwide level have been launched by the GlobalSepsis-Alliance and the Surviving Sepsis Campaign (SSC) to improve care and survival rates. Sepsis and, in particular, septic shock are serious illnesses usually requiring intensive care management, resulting in very high hospitalisation costs. Sepsis-related costs in U.S. hospitals surpass U.S. $24 billion annually, making it the most expensive disease to manage (Torio and Moore 2015). The diagnosis of sepsis is currently based on the 2016 Sepsis-3 definition (Singer et al. 2016) as an infection and a dysregulated reaction of the body characterised by organ failure.
Nosocomial Infections and Sepsis
Nosocomial infections, i.e., Hospital Acquired Infections (HAI), occur in 7-8% of hospitalised patients in Europe (Swissnoso) and even 56% of patients hospitalised in intensive care unit ICU (Vincent et al. 2020). The WHO estimated 1.4 million nosocomial infections in 2016 and forecasts 10 million deaths in 2050 (Dadgostar 2019). The main causes of nosocomial infections are bacterial antimicrobial resistance (AMR), lack of adherence to infection control and prevention procedures.
In an epidemiological study published in 2020 (Markwart et al. 2020), the proportion of nosocomial sepsis, i.e., hospital-acquired sepsis (HAS) among all hospital-treated sepsis cases, was 23.6% (95% CI 17–31.8%). In the ICU, 24.4% (95% CI 16.7–34.2%) of cases of sepsis with organ dysfunction were acquired during ICU stay, and 48.7% (95% CI 38.3–59.3%) had a hospital origin. The pooled hospital incidence of HAS with organ dysfunction per 1000 patients was 9.3 (95% CI 7.3–11.9%). Mortality of ICU patients with HAS with organ dysfunction was 52.3% (95% CI 43.4–61.1%). The article concludes, "there is an urgent need to improve the implementation of global and local infection prevention and management strategies to reduce its high burden among hospitalized patients”.
In this context, the first recommendation in the latest SSC 2021 guidelines (Evans et al. 2021) propose, "for hospitals and health systems, we recommend sepsis screening for acutely ill and high-risk patients (Strong recommendation, moderate quality of evidence)”. But it's not defined how screening should be carried out. Prophylactic administration of broad-spectrum antibiotics is clearly not recommended to avoid exacerbating the major problem of AMR.
Screening of Nosocomial Sepsis for Acutely Ill and High-Risk Patients
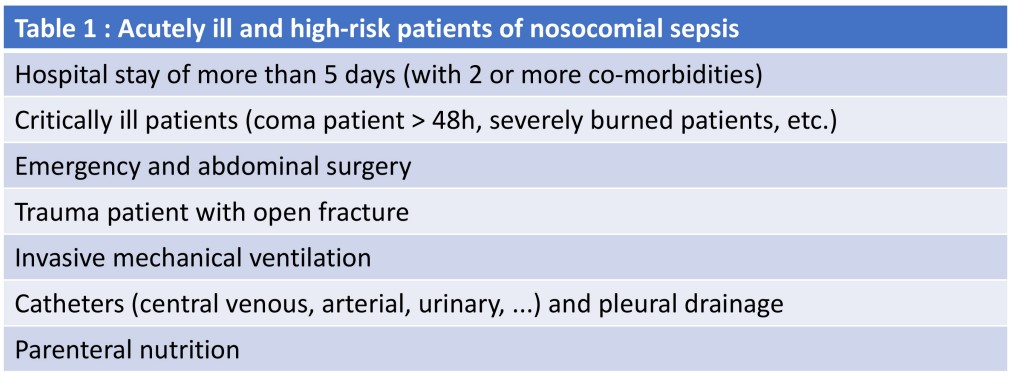
The definition of acutely ill and highrisk patients is not specified in the SSC 2021 guidelines, but it is often suggested (Table 1) that it is those with an expected hospital stay of more than five days (with two or more co-morbidities), critically ill patients, coma patient >48h, severely burned patients, emergency and abdominal surgery, trauma patient with open fracture, patient with invasive mechanical ventilation, catheters (central venous, arterial, urinary, ...), pleural drainage and parenteral nutrition (Farinas-Alvarez et al. 2000; Appelgren et al. 2001).
Current nosocomial sepsis screening tools are designed to promote early identification, maybe even before symptoms (pre-symptomatic diagnosis of nosocomial sepsis) and consist of manual methods or automated use (with or without artificial intelligence) of the electronic health record (EHR), biomarkers, and/or transcriptomic technology.
Electronic Health Record
According to SSC 2021 guidelines (Evans et al. 2021), “there is wide variation in diagnostic accuracy of these tools with most having poor predictive values, although the use of some was associated with improvements in care processes. A variety of clinical variables and tools are used for sepsis screening, such as systemic inflammatory response syndrome (SIRS) criteria, vital signs, signs of infection, quick Sequential Organ Failure Score (qSOFA) or Sequential Organ Failure Assessment (SOFA) criteria, National Early Warning Score (NEWS), or Modified Early Warning Score (MEWS). Machine learning may improve the performance of screening tools, and in a meta-analysis of 42,623 patients from seven studies for predicting hospital-acquired sepsis, the pooled area under the receiving operating curve (SAUROC) (0.89; 95% CI, 0.86−0.92); sensitivity (81%; 95% CI, 80−81), and specificity (72%; 95% CI, 72−72) was higher for machine learning than the SAUROC for traditional screening tools such as SIRS (0.70), MEWS (0.50), and SOFA (0.78). Screening tools may target patients in various locations, such as in-patient wards, emergency departments, or ICUs. A pooled analysis of three RCTs did not demonstrate a mortality benefit of active screening (RR, 0.90; 95% CI, 0.51−1.58)”.
Biomarkers
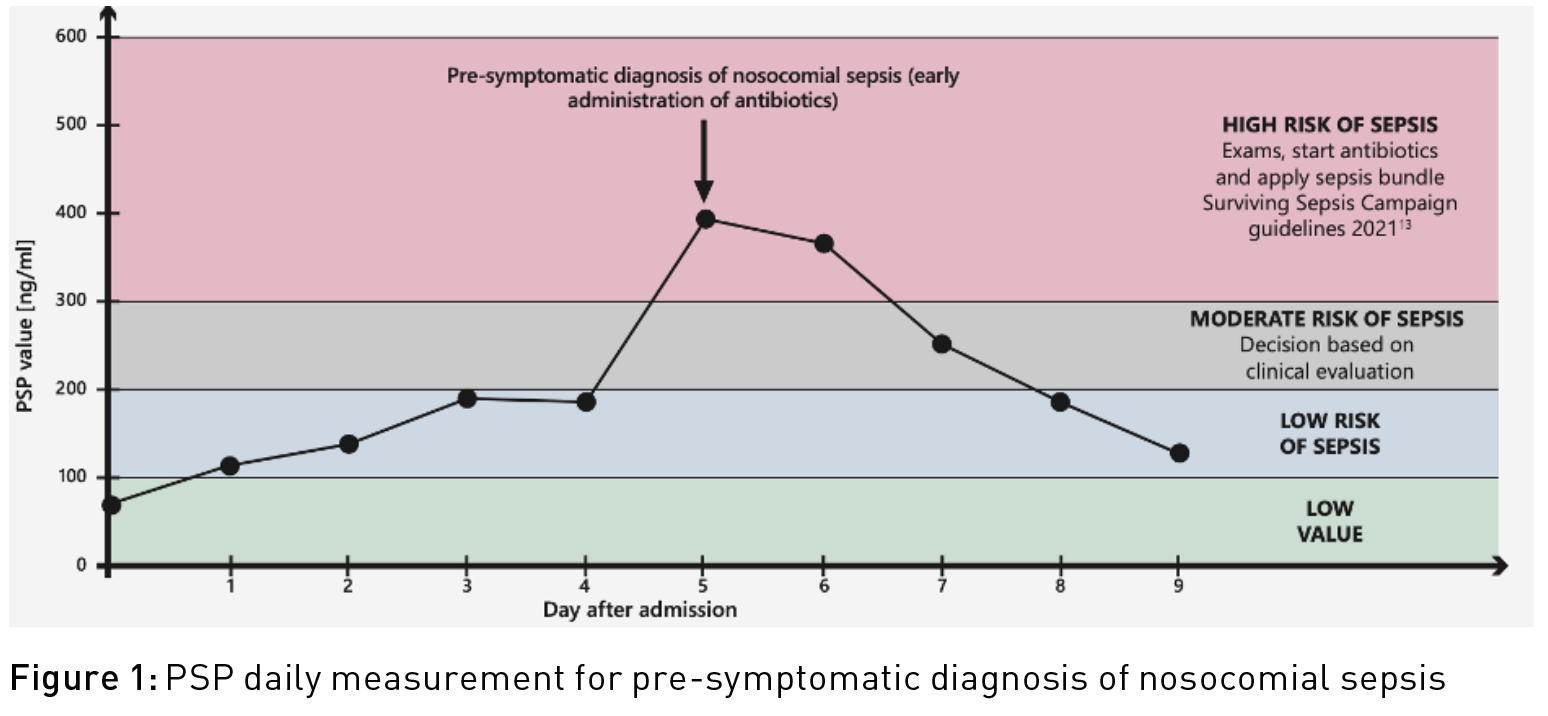
Numerous studies have been carried out on a wide range of biomarkers (Procalcitonin PCT, C-reactive protein CRP, presepsin, leukocytes, Interleukin-6, monocyte distribution width, etc.) to screen for early sepsis when first symptoms appear. Several studies (Klein et al. 2020; Klein et al. 2015; Pugin et al. 2021) have shown that the Pancreatic Stone Protein (PSP) can detect sepsis up to three to five days before the first symptoms appear (pre-symptomatic diagnosis of nosocomial sepsis). In a cohort of 90 severely burned patients (Klein et al. 2020), “PSP differentiated between sepsis, infection, and sterile inflammation from day 3 onward with an area under the curve of up to 0.89 (P < 0.001)”. In an unselected population of cardiac surgery patients (Klein et al. 2015), “post-operative serum PSP levels were significantly associated with the presence of infection in both the on-pump and off-pump setting.” A prospective multi-centre study published in 2021 in Critical Care (Pugin et al. 2021) shows that “serial PSP measurement demonstrated an increase of this marker the days preceding (up to 3-5 days) the onset of signs necessary to clinical diagnose sepsis”. From then on, numerous centres in dozens of countries proposed to measure PSP daily in acutely ill and high-risk patients to assess the risk of sepsis (Figure 1).
As shown by two literature reviews, from 2019 (13 studies) (Eggimann et al. 2019) and from February 2022 (Fidalgo et al. 2022) (23 studies), PSP is confirmed as an innovative tool for early detection of sepsis, infection diagnosis, and to predict severity and mortality. PSP is a 16 kDs C-type lectin protein produced mostly by the pancreas and the intestine and is a damage-associated molecular pattern DAMPs. PSP is measured in seven minutes from a drop of capillary, venous or arterial whole blood at the point-of-care POC (CE certified IVDR 2022, Intended for use: Risk of sepsis, abioSCOPE®, Abionic SA, Epalinges, Switzerland). A 2021 independent U.S. economic study (Schneider et al. 2022) shows that the use of PSP could save the U.S. healthcare system U.S. $7 billion a year.
Transcriptomic
There are a few studies (Bodinier et al. 2023; Lukaszewski et al. 2022) using transcriptomic technology for pre-symptomatic diagnosis of nosocomial infection and sepsis. However, this complex and expensive technology can currently only be used in clinical research contexts, with unsatisfactory results in everyday practice.
Conclusion
Hospital and ICU nosocomial sepsis screening for acutely ill and high-risk patients is strongly recommended (moderate quality of evidence) by the latest SSC 2021 guidelines (Evans et al. 2021). A specific tool for pre-symptomatic diagnosis of nosocomial sepsis is not currently recommended, but daily measurement of PSP has been shown to diagnose nosocomial sepsis three to five days before the onset of symptoms (Pugin et al. 2021). Compared to the automated study of the patient's electronic health record EHR by complex algorithms (with or without artificial intelligence) and too slow and expensive transcriptomic technology, the cheap PSP assay can be performed at the patient's bedside in seven minutes from 50 ul of whole blood and with major savings potential for healthcare systems (7 billion/ year in the U.S.) (Schneider et al. 2022).
Disclaimer
Point-of-View articles are the sole opinion of the author(s) and they are part of the ICU Management & Practice Corporate Engagement or Educational Community Programme.




















