Based on findings of a new study, changes in brain connections visible on MRI could represent an imaging biomarker of Alzheimer's disease, the most common form of dementia. For this study, researchers examined the brain's structural connectome, a map of white matter tracts that carry signals between different areas of the brain.
"The structural connectome provides us with a way to characterise and measure these connections and how they change through disease or age," said study co-author Jeffrey W. Prescott, MD, PhD, radiology resident at Duke University Medical Center in Durham, N.C. The study was presented at the annual meeting of the Radiological Society of North America (RSNA) in Chicago, Illinois.
The research team evaluated results from 102 patients enrolled in a national study called the Alzheimer's Disease Neuroimaging Initiative (ADNI) 2. The patients had undergone diffusion tensor imaging (DTI), an MRI technique that assesses the integrity of white matter tracts in the brain by measuring how easy it is for water to move along them. Water is known to move along the defined physical connections between regions in the brain, which makes DTI an effective tool for evaluating the structural connectome, Dr. Prescott explained.
The Duke team correlated changes in the structural connectome with results from florbetapir positron emission tomography (PET) imaging, a technique that measures the amount of beta amyloid plaque in the brain. A high florbetapir uptake corresponds with greater amounts of the protein.
The results indicated a strong link between florbetapir uptake and decreases in strength of the structural connectome in each of the five regions of the brain studied. As Dr. Prescott noted, "This study ties together two of the major changes in the Alzheimer's brain—structural tissue changes and pathological amyloid plaque deposition—and suggests a promising role for DTI as a possible diagnostic adjunct."
The findings suggest DTI may offer a role in assessing brain damage in early Alzheimer's disease and monitoring the effect of new therapies. Some five million Americans are affected by the disease and the number is expected to hit 14 million by 2050, according to the Centers for Disease Control and Prevention.
Experts say preventive treatments may be most effective before Alzheimer's disease is diagnosed, such as when a person is suffering from mild cognitive impairment (MCI), a decline in cognitive skills that is noticeable but not severe enough to affect independent function. Previous efforts at early detection have focused on beta amyloid, a protein found in abnormally high amounts in the brains of people with Alzheimer's disease.
"Traditionally, Alzheimer's disease is believed to exert its effects on thinking via damage to the brain's grey matter, where most of the nerve cells are concentrated," said senior author Jeffrey R. Petrella, MD, professor of radiology at Duke. "This study suggests that amyloid deposition in the grey matter affects the associated white matter connections, which are essential for conducting messages across the billions of nerve cells in the brain, allowing for all aspects of mental function."
Dr. Prescott added: "We suspect that as amyloid plaque load in the grey matter increases, the brain's white matter starts to break down or malfunction and lose its ability to move water and neurochemicals efficiently."
The research team plans to continue studying this cohort of patients over time to better understand how the disease evolves in individual patients. They also want to integrate functional imaging into their work to learn about how the relationship between function and structure is affected with increasing amyloid burden.
Other members of the research team are Kingshuk R. Choudhury, PhD, and P. Murali Doraiswamy, MD.
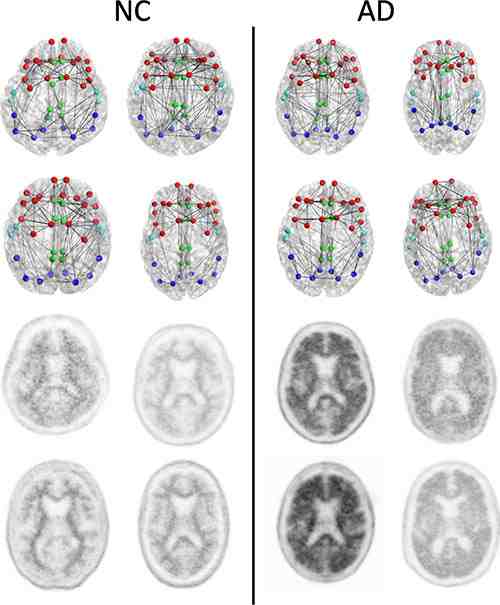
Figure 1. Structural connectomes (top two rows) and corresponding florbetapir PET images (bottom two rows) in four patients with normal cognition (NC) with the lowest whole cortex amyloid burden (left) and the four patients with AD with the highest whole cortex amyloid burden (right) focused on the composite regions used in connectome versus amyloid analysis. Nodes represent the centroids of the FreeSurfer parcellations in the frontal (red), cingulate (green), temporal (light blue), and parietal (dark blue) regions. This is merely a schematic intended to show the concepts and is not intended to show any visually discernible generalisable difference between the patients with NC and those with AD. Structural network metrics provide more sensitive information about the connectome than are apparent through visualisation alone.
Source: RSNA
Image Credit: RSNA
"The structural connectome provides us with a way to characterise and measure these connections and how they change through disease or age," said study co-author Jeffrey W. Prescott, MD, PhD, radiology resident at Duke University Medical Center in Durham, N.C. The study was presented at the annual meeting of the Radiological Society of North America (RSNA) in Chicago, Illinois.
The research team evaluated results from 102 patients enrolled in a national study called the Alzheimer's Disease Neuroimaging Initiative (ADNI) 2. The patients had undergone diffusion tensor imaging (DTI), an MRI technique that assesses the integrity of white matter tracts in the brain by measuring how easy it is for water to move along them. Water is known to move along the defined physical connections between regions in the brain, which makes DTI an effective tool for evaluating the structural connectome, Dr. Prescott explained.
The Duke team correlated changes in the structural connectome with results from florbetapir positron emission tomography (PET) imaging, a technique that measures the amount of beta amyloid plaque in the brain. A high florbetapir uptake corresponds with greater amounts of the protein.
The results indicated a strong link between florbetapir uptake and decreases in strength of the structural connectome in each of the five regions of the brain studied. As Dr. Prescott noted, "This study ties together two of the major changes in the Alzheimer's brain—structural tissue changes and pathological amyloid plaque deposition—and suggests a promising role for DTI as a possible diagnostic adjunct."
The findings suggest DTI may offer a role in assessing brain damage in early Alzheimer's disease and monitoring the effect of new therapies. Some five million Americans are affected by the disease and the number is expected to hit 14 million by 2050, according to the Centers for Disease Control and Prevention.
Experts say preventive treatments may be most effective before Alzheimer's disease is diagnosed, such as when a person is suffering from mild cognitive impairment (MCI), a decline in cognitive skills that is noticeable but not severe enough to affect independent function. Previous efforts at early detection have focused on beta amyloid, a protein found in abnormally high amounts in the brains of people with Alzheimer's disease.
"Traditionally, Alzheimer's disease is believed to exert its effects on thinking via damage to the brain's grey matter, where most of the nerve cells are concentrated," said senior author Jeffrey R. Petrella, MD, professor of radiology at Duke. "This study suggests that amyloid deposition in the grey matter affects the associated white matter connections, which are essential for conducting messages across the billions of nerve cells in the brain, allowing for all aspects of mental function."
Dr. Prescott added: "We suspect that as amyloid plaque load in the grey matter increases, the brain's white matter starts to break down or malfunction and lose its ability to move water and neurochemicals efficiently."
The research team plans to continue studying this cohort of patients over time to better understand how the disease evolves in individual patients. They also want to integrate functional imaging into their work to learn about how the relationship between function and structure is affected with increasing amyloid burden.
Other members of the research team are Kingshuk R. Choudhury, PhD, and P. Murali Doraiswamy, MD.
Figure 1. Structural connectomes (top two rows) and corresponding florbetapir PET images (bottom two rows) in four patients with normal cognition (NC) with the lowest whole cortex amyloid burden (left) and the four patients with AD with the highest whole cortex amyloid burden (right) focused on the composite regions used in connectome versus amyloid analysis. Nodes represent the centroids of the FreeSurfer parcellations in the frontal (red), cingulate (green), temporal (light blue), and parietal (dark blue) regions. This is merely a schematic intended to show the concepts and is not intended to show any visually discernible generalisable difference between the patients with NC and those with AD. Structural network metrics provide more sensitive information about the connectome than are apparent through visualisation alone.

Source: RSNA
Image Credit: RSNA
Latest Articles
MRI, PET, RSNA 2014, #RSNA14, Alzheimer’s disease, connectome
Based on findings of a new study, changes in brain connections visible on MRI could represent an imaging biomarker of Alzheimer's disease, the most common...