The global demand for imaging tests to assess cancer is increasing, but there's a shortage of imaging specialists, particularly in low and middle-income countries. This leads to delays in scan reporting and patient management. Artificial intelligence (AI) using deep neural networks (DNNs) could help handle high scan volumes while maintaining diagnostic accuracy and providing results more rapidly. In oncological imaging, AI has shown promise in tasks like detecting hypermetabolic tumour sites on PET scans, which is crucial for cancer diagnosis. A recent article published in the Lancet Digital Health discusses the development and evaluation of a DNN algorithm called Lymphoma Artificial Reader System (LARS) that automatically classifies [18F]FDG-PET-CT scans of lymphoma patients. The algorithm was trained on a large dataset of weakly labelled scans and showed promising accuracy, sensitivity, and specificity for identifying hypermetabolic tumour sites.
Dual-Centre Study Design and Dataset Description
This retrospective dual-centre study conducted at Memorial Sloan Kettering Cancer Centre (MSK) in New York, USA, and the Medical University of Vienna (MUV) in Austria included patients with biopsy-proven [18F]FDG-avid lymphomas who had undergone whole-body [18F]FDG-PET-CT for routine purposes. Patients were selected based on specific criteria and exclusion factors. At MSK, data from patients with the four most common [18F]FDG-avid lymphoma subtypes between Jan 1, 2010, and Jan 31, 2021, were collected. At MUV, external test data were obtained from patients with [18F]FDG-avid lymphomas. The study involved a total of 19,721 PET-CT scans from 5,466 patients at MSK, with exclusions narrowing down the dataset to 16,583 scans from 5,072 patients for training and testing. Additionally, 1,000 PET-CT scans from 503 patients were included in the external test dataset from MUV. The study utilised specific imaging protocols and split the MSK dataset into 80% training data and 20% held-out test data for evaluation.
Significance of the Study in Applying Deep Learning to PET-CT Imaging
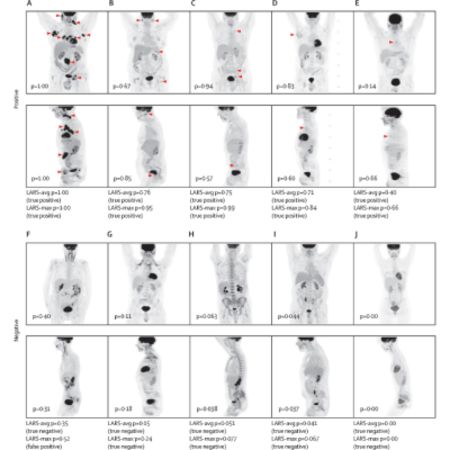
The study is the largest so far to apply deep learning to PET-CT imaging in oncology. Notably, the prior largest study focused on tumour segmentation and involved only 3,664 scans. The algorithm developed, Lymphoma Artificial Reader System (LARS), achieved an impressive AUC of 0.95 in both internal and external test datasets, demonstrating a balanced accuracy of 87-91% when utilizing PET Maximum Intensity Projections (MIPs) as input. Despite differences in imaging protocols and patient populations between the two participating centres, LARS exhibited consistent performance. This consistency suggests its potential generalizability across diverse clinical settings, scanners, and lymphoma subtypes. Moreover, LARS showed promise for application in other FDG-avid cancers beyond lymphoma.
Clinical Applications and Potential Impact of LARS
The study outlined several potential clinical applications for LARS. It could be employed to rule out the presence of hypermetabolic tumour sites, particularly in post-treatment settings, enabling the automatic generation of preliminary reports to expedite patient management. Additionally, LARS could serve as a valuable second reader, enhancing diagnostic accuracy and confidence, and potentially mitigating the impact of high scan volumes on radiologists and nuclear medicine physicians. Furthermore, due to its high specificity, LARS could function as a decision-support tool in equivocal cases, aiding in the differentiation between tumour and non-tumour uptake. Various versions of LARS were evaluated, with PET MIP-based models proving to be particularly efficient and effective, offering faster computation times and lower energy consumption compared to 3D PET and CT-based models. However, the study acknowledged several limitations, including the exclusion of unresolved cases and the heterogeneity of lymphoma subtypes, which may have influenced model performance.
The study underscores the potential of deep learning algorithms like LARS to improve the interpretation of PET-CT scans in lymphoma and other FDG-avid cancers. Prospective evaluation is warranted to further assess its clinical utility and impact on patient care.
Source & Image Credit: The Lancet Digital Health