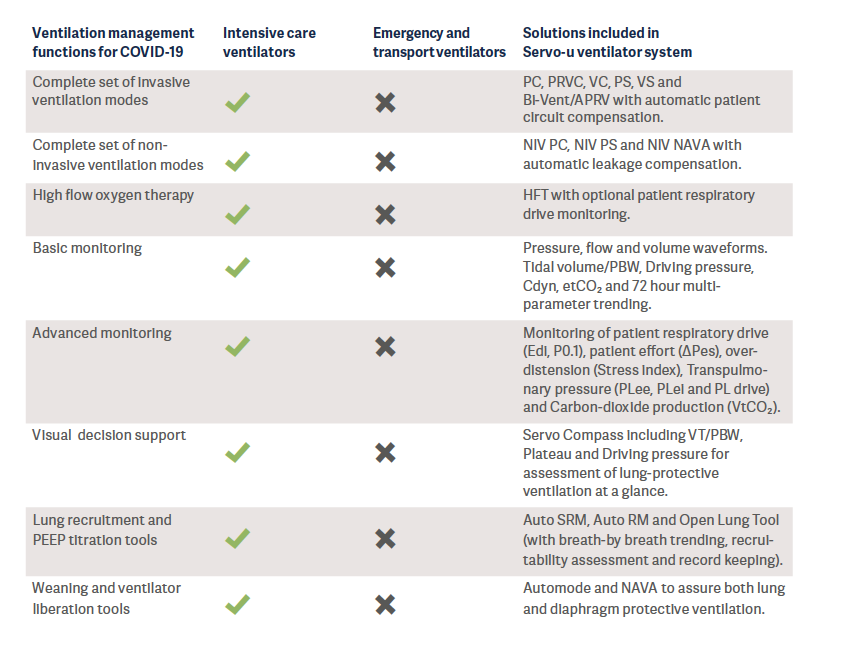
Summary & recommendations regarding ventilators
Intensive care ventilators are normally found in the hospital Intensive Care Unit. This category of ventilators support lung protective ventilation recommended by several international guidelines and the World Health Organization (WHO).
An intensive care ventilator must be considered as the primary option during the monitoring and treatment of COVID-19 patients in the ICU.
Background
Mechanical ventilation is life saving for patients with respiratory failure and is a key component in the fight against the new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). It can also damage the lungs if the pressures or volumes are not carefully monitored and controlled1. Therefore, mechanical ventilation must be carefully applied in a balance between insufficient ventilation and ventilation-induced lung injury (VILI) – which has a strong impact on long-term outcome and disabilities.
Mechanical ventilation can either be invasive, via a tube in the airways, or non-invasive through a face mask or nasal prongs. However, in many cases the patient’s condition will require intubation, e.g., with severe lung injury or disease.
Transport & emergency ventilators with COVID-19
When the recently mass-produced ventilators from new entrants to the industry were reviewed, it was found that most of them had a functionality comparable to today’s transport or emergency ventilators (See Appendix for classification of ventilators). Transport and emergency ventilators are mainly used for the short term and for patients who are in stable condition. However, these ventilators unfortunately do not fulfill the technical specifications published by WHO – which describe the minimum requirements that invasive and non-invasive ventilators must comply with to ensure quality, safety and effectiveness when used for the management of COVID-192.
It is also well-known that these simpler ventilators cannot generate the precise air delivery needed to treat patients with acute respiratory distress syndrome (ARDS)3,4 and they are lacking decision support tools for lung protective ventilation. In a crisis situation, these ventilators are a better alternative compared to manual ventilation (“bagging’’), however they do not take the place of the intensive care ventilators.
ECRI, a worldwide organization that conducts independent medical device evaluations, clearly states that, in the case of shortages of intensive care ventilators during the COVID-19 pandemic, health care providers should select from available ventilation devices in the following suggested order of preference5:
1. Intensive care ventilators.
2. Advanced transport, sub-acute, and home care ventilators that have intensive care features and are capable of treating patients with ARDS.
3. Anesthesia machines. See ECRI Alert S0397: [COVID-19]. Anesthesia units can be repurposed to provide ventilatory support for critically ill patients, as long as precautions are taken.
4. Basic transport, emergency, and home care ventilators.
Non-invasive ventilators with COVID-19
The most common diagnosis6 in severe COVID-19 patients is severe pneumonia associated with sepsis and ARDS7.
The latest studies about mechanically-ventilated patients with COVID-19 show that non-invasive ventilators alone will not be sufficient for COVID-19 patients, since these type of ventilation devices are not designed for invasive ventilation of a critically ill, complex patient and most of the COVID-19 patients require invasive ventilation during their ICU stay8.
Intensive care ventilators with COVID-19
The first step to optimizing mechanical ventilation for patients’ conditions and needs in the ICU is to use high performance ventilators9 such as Getinge Servo-u. The Getinge Servo-u uses built-in sensors, microprocessors, and intelligent software to adequately provide and moni- tor the target ventilation and automatically adjust the ventilator parameters based upon patients’ needs. It is well-known that mechanical ventilation is life saving for patients with respiratory failure, but it can also damage the lungs if the pressures or volumes are not carefully controlled and monitored.
Well-recognized, key opinion leader in mechanical ventila- tion, Luciano Gattinoni, also focused on the importance of monitoring lung mechanics (e.g., respiratory system com- pliance, esophageal and transpulmonary pressure) to iden- tify the different phenotypes of COVID-1910, and individualize the treatment accordingly.
In the figure below, drivers and interrupters of progressive lung injury in COVID-19 infection, are identified.
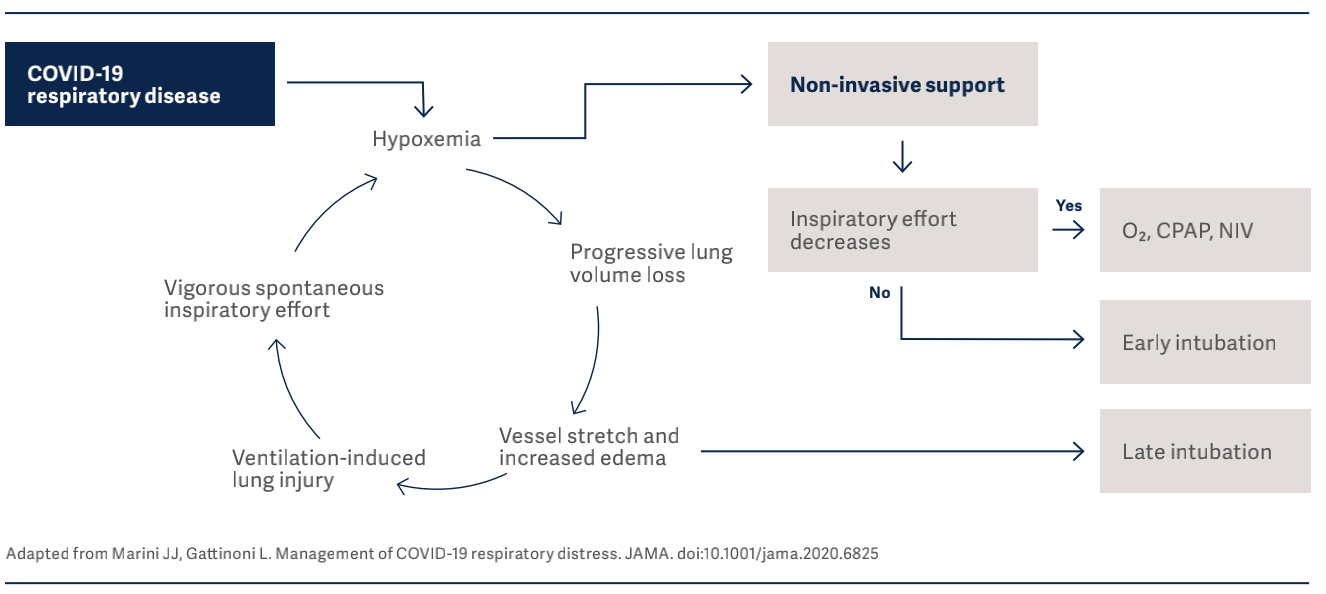
It is vital to know that the clinical presentation of COVID-19 patients can change over time, and monitoring of esophageal pressure swings is of extreme importance in these patients to detect risk of lung injury10. In their interim guidance document11, WHO also strongly recom- mends implementing lung protective ventilation strategies for the management of critical COVID-19.
Therefore, intensive care ventilators play a critical role during the COVID-19 pandemic since they:
• Can support clinicians in every phase of COVID-19, from non-invasive (NIV) to invasive ventilation modes if the patient’s condition worsens. Clinicians do not have to triage the ventilation devices at their disposal, matching the device capabilities with the severity of the patient illness due to ventilator shortages.
• Can generate the precise gas delivery needed to treat patients with ARDS (acute lung injury).
• Are able to adequately provide and monitor the target ventilation, and automatically adjust the ventilatory parameters, based on the patient’s needs in order to protect the lungs and prevent lung injury.
• Offer advanced monitoring tools such as P0.1, Edi, ΔPes, PL, and transpulmonary pressure monitoring, which allows the clinician to understand the effects of mechanical ventilation in real time and adjust the ventilatory parameters on the basis of the patient’s condition and needs.
• Provide early alerts and alarms to warn clinicians of a potentially harmful situation, or if the patient becomes distressed or even crashes. Please note that the alarm capabilities for emergency, transport, and home care ventilators can be very limited4.
• Ability to deliver collaborative therapy – offer nebulized drugs through a closed circuit state-of-the art nebulizer system.
• Ability to remove the user interface, keep it outside the room in order to reduce exposure and the use of PPE.
• Have the advanced weaning modalities, which will help the patient liberate from mechanical ventilation faster.
Getinge’s Servo ventilators support clinicians with ad- vanced lung protective tools – which helps to identify in- jurious ventilation and to diagnose clinical problems.
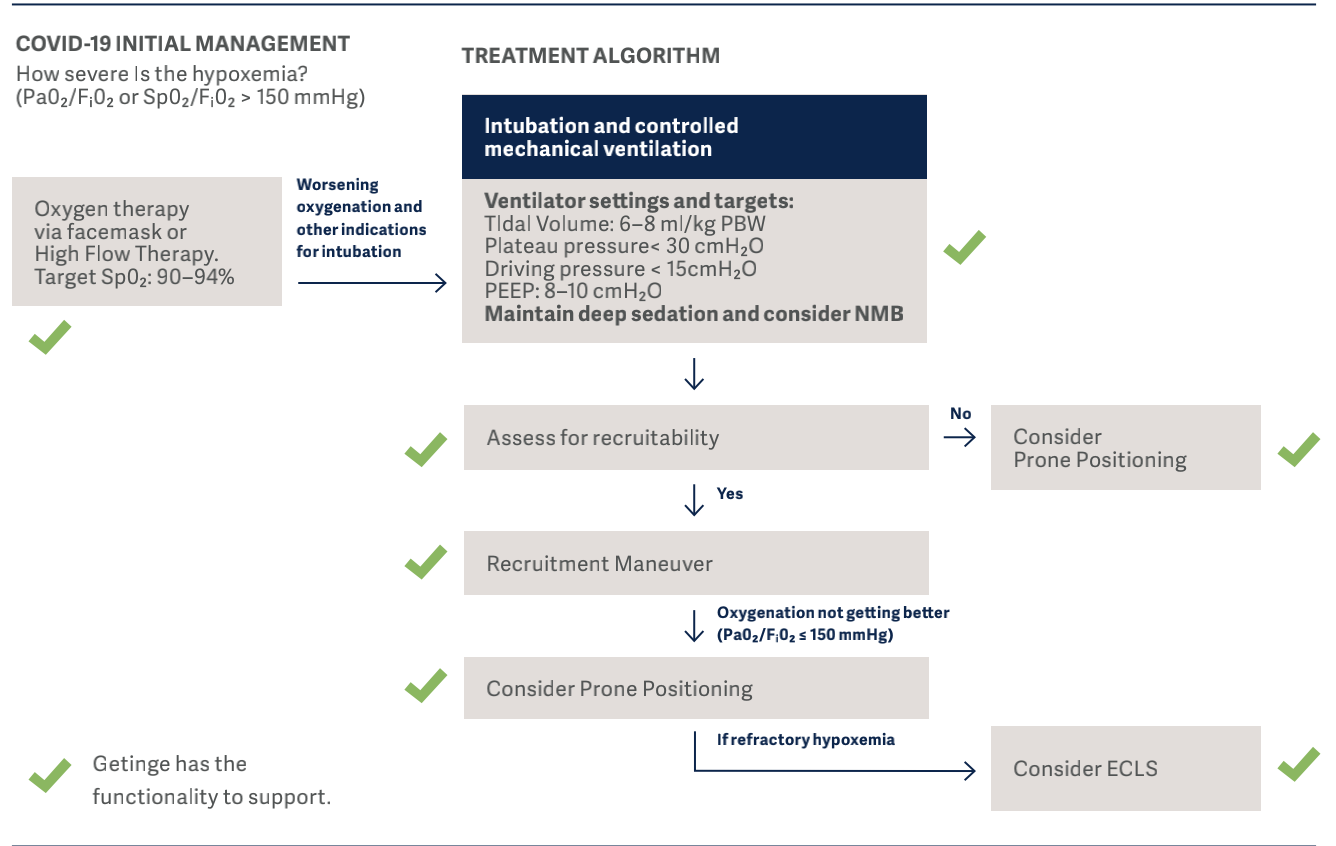
In the flowchart below, the clinical guidelines for COVID-19, which has been developed by representatives of the University of Toronto Interdepartmental Division of Critical Care Medicine, are demonstrated12. Using these guidelines we are able to show where Getinge can support or offers a solution.
References
1. Dreyfuss D, Basset G, Soler P, Saumon G (1985) Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 132:880–884
2. Technical specifications for invasive and non-invasive ventilators for COVID-19: interim guidance: https://apps.who.int/iris/bitstream/ handle/10665/331792/WHO-2019-nCoV-Clinical-Ventilator_Specs-2020.1-eng.pdf?sequence=1&isAllowed=y
3. Chipman DW, Caramez MP, Miyoshi E et al. Performance Comparison of 15 Transport Ventilators Respir
Care 2007;52(6): 740–751.
4. ECRI Exclusive Special Report - S0404: [COVID-19] Philips— Respironics E30 Ventilators: ECRI Assessment of Emergency Use Authorization Device
5. ECRI Exclusive Special Report - S0398 : [COVID-19] Shortages of Intensive Care Ventilators—Strategies for Mitigation. Medical Device Special Report
6. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance 13 March 2020. WHO reference number: WHO/2019-nCoV/clinical/2020.4
7. Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol 2020 Apr 15 [Epub ahead of print]. doi: 10.1002/jmv.25884.
8. Ziehr DR, Alladina J, Petri CR et al. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. AJRCCM Articles in Press. Published April 29, 2020 as 10.1164/rccm.202004-1163LE.
9. Govoni L, Dellaca RL, Penuelas O, et al. Actual performance of mechanical ventilators in ICU: a multicentric quality control study. Medical Devices: Evidence and Research 2012:5 111–119
10. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatment for different phenotypes? (2020) Intensive Care Medicine; DOI: 10.1007/s00134-020-06033-2
11. Analysis of the technologies required for clinical management of COVID-19 patients https://www.who.int/emergencies/diseases/ novel-coronavirus-2019/technical-guidance/patient-management.
https://www.criticalcare.utoronto.ca/covid-19-resources
Appendix - Classification of ventilators
• Intensive care ventilators: Ventilators designed for use in the ICU.
• Home care ventilators: Ventilators used for long-term ventilation in the patient’s home environment.
• Sub-acute ventilators: Ventilators that are desingned for use outside of the ICU, through either non-invasive ventilation, or ventilation of stable patients. This includes step-down wards, general wards, and skilled nursing facilities.
• Emergency & Transport ventilators: Ventilators used in emergency situations both within the hospital and externally (mainly for short term).






