ICU Management & Practice, Volume 24 - Issue 2, 2024
Emerging evidence highlights the potential advantages of employing anti-inflammatory therapies like macrolide antibiotics and corticosteroids in managing severe community-acquired pneumonia (CAP). Although their use remains a subject of debate, recent findings indicate improved clinical outcomes, and support the adoption of a tailored approach that emphasises personalised medicine strategies to optimise treatment efficacy and minimise adverse effects.
Introduction
Community-acquired pneumonia (CAP) remains a significant healthcare challenge and contributes substantially to patient morbidity and mortality worldwide (GBD 2017 Causes of Death Collaborators 2018). It stands out as a prevalent cause of respiratory failure and admissions to intensive care units (ICUs) (Cavallazzi et al. 2020). Within the spectrum of CAP, a subset of the population experiences severe disease, classified as severe CAP based on criteria established by the American Thoracic Society and Infectious Disease Society of America (ATS/IDSA) (Metlay et al. 2019; Dremsizov et al. 2006). Notably, patients meeting severe CAP criteria could face in-hospital mortality up to 17%, with a 1-year mortality rate as high as 50% (Cavallazzi et al. 2020; Marrie and Shariatzadeh 2007; Riley, Aronsky, and Dean 2004).
The treatment approach to CAP is focused on early identification, triage to appropriate levels of care, and prompt administration of effective antibiotics (Metlay et al. 2019). However, controversies persist regarding the optimal choice of therapy and the role of adjunctive anti-inflammatory treatments, such as the addition of macrolide antibiotics and systemic corticosteroids.
Given that pneumonia, a common cause of sepsis, is associated with a dysregulated immune response leading to systemic inflammation—a cornerstone of sepsis pathobiology—the consideration of anti-inflammatory agents is logical.(Angus and van der Poll 2013) Despite ongoing debates fuelled by varying evidence, refining the role of macrolides and corticosteroids in CAP management is crucial for advancing treatment strategies and improving clinical outcomes. Nonetheless, in the transition towards an era of personalised medicine, it is important to acknowledge that therapies may not follow a "one-size-fits-all" approach, emphasising the need for individualised treatment strategies.
In this review, we delve into the specific roles of macrolide antibiotics and systemic corticosteroids in addressing severe CAP. We examine the most recent guidelines and evidence regarding their use and highlight individual considerations crucial in the decision-making process when contemplating the administration of these therapies.
The Role of Macrolide Antibiotics
Macrolide antibiotics have been a recommended component of combination therapy in the treatment of both CAP and severe CAP for over a decade (Mandell et al. 2007; Metlay et al. 2019; Martin-Loeches et al. 2023). Despite accumulating evidence supporting their clinical efficacy, their usage remains a topic of debate. In 2019, the ATS and IDSA issued updated guidelines advocating for the empirical administration of beta-lactam antibiotics combined with macrolides or fluoroquinolones for patients with severe CAP (Metlay et al. 2019). Similarly, the most recent guidelines released by the European Respiratory Society (ERS), the European Society of Intensive Care Medicine (ESICM), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and the Latin American Thoracic Association (ALAT), also recommended the combination of beta-lactams with either a macrolide or fluoroquinolones with a preference for macrolide antibiotics, for those with severe CAP (Martin-Loeches et al. 2023).
Mortality and Clinical Benefits
The recommendations for macrolide antibiotics in guidelines are largely informed by observational studies demonstrating survival benefits when they are added to beta-lactams (García Vázquez et al. 2005; Lodise et al. 2007; Martínez et al. 2003; Metersky et al. 2007; Restrepo et al. 2009; Martin-Loeches et al. 2010; Sligl et al. 2014). In a matched case-controlled study of two prospective cohorts in Europe with 80 patients diagnosed with CAP, ICU mortality had an observed 80% reduction in the odds of mortality when combination therapy, including a macrolide, was used (Gattarello et al. 2014).
To further evaluate the clinical effects of combination antibiotics, including macrolides, randomised controlled trials (RCT) have been conducted. However, they have yielded conflicting results and faced notable limitations, with many not focusing on those with severe CAP. One study was the Community-Acquired Pneumonia - Study on the Initial Treatment with Antibiotics of Lower Respiratory Tract Infections (CAP-START) that compared beta-lactam monotherapy to beta-lactam plus macrolide, or quinolone therapy. While the study concluded that beta-lactam monotherapy was non-inferior to quinolone or beta-lactam plus macrolide therapy for 90-day mortality, important criticisms included nearly 25% of patients without radiographic confirmation of pneumonia, off-protocol macrolide therapy in the monotherapy groups (38.7%), and overall lower disease severity of the patient population, most notably the exclusion of ICU patients (Postma et al. 2015). Additionally, the incidence of atypical bacteria and resistant Streptococcus pneumoniae was relatively low in this population, compared to other regions of the world.
In another non-inferiority RCT, beta-lactam monotherapy was compared with beta-lactam plus macrolide therapy in hospitalised patients with moderately severe CAP. The study did not find non-inferiority of monotherapy when compared to patients with combination therapy in terms of improvement in clinical stability at day 7. 90-day mortality, a secondary outcome, also did not differ between the two arms (Garin et al. 2014). Furthermore, patients who had microbiological evidence of atypical infection and those with a higher pneumonia severity index (PSI IV) had delayed clinical stability on monotherapy (Garin et al. 2014).
In response to conflicting evidence, a more recent multicentre RCT known as the Anti-inflammatory Action of Oral Clarithromycin in Community-acquired Pneumonia (ACCESS) trial was conducted and focused on those with severe CAP. This trial evaluated the adjunctive use of clarithromycin with beta-lactam and revealed a statistically significant improvement in early clinical response compared to placebo (Giamarellos-Bourboulis et al. 2024). Early clinical response was defined as a composite of at least a 50% decrease in respiratory symptom severity, at least a 30% decrease in sequential organ failure assessment (SOFA) score, or favourable procalcitonin (PCT) kinetics (at least an 80% decrease from baseline) on Day 4 of trial assessment. While mortality was not a primary endpoint, the study observed trends toward improvement at various time points throughout the study duration, and a post-hoc analysis demonstrated a mortality benefit in the clarithromycin group at the end-of-treatment visit. The study was meticulously designed and included patients with more severe community-acquired pneumonia (CAP) who met stringent clinical and radiographic criteria. Exclusion criteria included patients who had received macrolide antibiotics, corticosteroids, or anti-cytokine treatment, had a QTc interval greater than 500 milliseconds or had a diagnosis of coronavirus disease 2019 (COVID-19). This trial contributes robust evidence in support of combination therapy, particularly involving macrolide antibiotics, underscoring their utility in the management of severe CAP.
Beyond Antimicrobial Effects
The clinical benefits of macrolide antibiotics are multifaceted and consist of anti-inflammatory and immunomodulatory mechanisms, improved host-pathogen interactions, as well as providing antimicrobial coverage against atypical bacterial pathogens. This is exemplified in their use as adjunct immune-modulating therapies in respiratory diseases such as chronic obstructive pulmonary disease (COPD) and bronchiectasis, where they reduce the potential for exacerbations (Yamaya et al. 2012; Kelly et al. 2018).
In pneumonia, the anti-inflammatory and immunomodulating benefits are supported both clinically and biologically. Clinically, this is evident in the continued efficacy of macrolides despite the rise in confirmed resistance in pathogens over time. For example, the prevalence of macrolide resistance in S. pneumoniae increased from 18% in 1998 to approximately 30% in 2019 in the United States, with higher rates of resistance in Europe and Asia (Hoban et al. 2001; Song et al. 2004). Despite this, observational studies have consistently demonstrated mortality benefits with macrolide combination therapy spanning this period (García Vázquez et al. 2005; Lodise et al. 2007; Martínez et al. 2003; Metersky et al. 2007; Restrepo et al. 2009; Gattarello et al. 2014).
To further illustrate the added efficacy of macrolide antibiotics, a study involving 237 patients diagnosed with CAP and sepsis revealed a reduction in mortality associated with the addition of macrolide in multivariate analysis, even in the presence of confirmed macrolide-resistant organisms (Restrepo et al. 2009). Furthermore, clinical benefits in the face of resistant organisms have been reaffirmed in patients with ventilator-associated pneumonia (VAP), where gram-negative pathogens predominated and would not have been expected to be treated by macrolides. In a double blinded RCT with 200 patients diagnosed with sepsis and VAP, patients who received clarithromycin 1 gram for 3 days exhibited a shorter time to VAP resolution and liberation from mechanical ventilation.
Biologically, macrolides can exert various effects. From the perspective of host interactions, they can help fortify the airway epithelium, enhancing its resilience against external injury. Studies conducted in vitro have shown that macrolide antibiotics can bolster transepithelial electrical resistance by modulating the processing of tight junction proteins; this helps prevent fluid and electrolyte leakage and reinforces protective barriers (Song et al. 2004).In addition, macrolides have been shown to influence mucus composition and promote their clearance (Asgrimsson et al. 2006; Tagaya et al. 2002). Moreover, macrolides also have the capability to alter biofilm structure by inhibiting polysaccharide synthesis and suppress quorum sensing (Ichimiya, Yamasaki, and Nasu 1994; Wozniak and Keyser 2004; Ichimiya et al. 1996) This is observed not only in Pseudomonal infections but also in Haemophilus influenzae and Staphylococcus epidermidis (Starner et al. 2008; Yasuda et al. 1994; Wozniak and Keyser 2004).
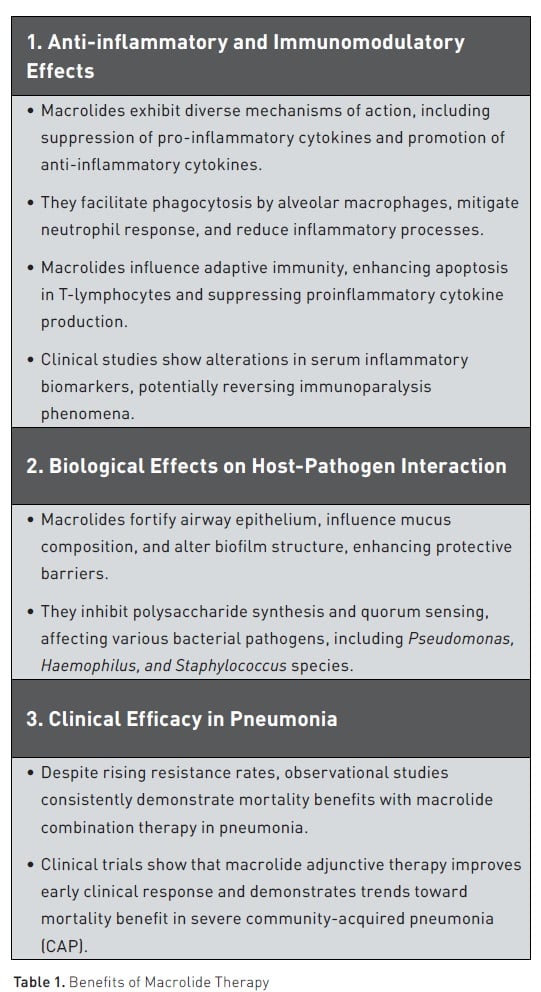
From an anti-inflammatory and immunomodulatory perspective, macrolides exhibit a diversity of mechanisms of action (Table 1). They have been found to suppress the synthesis of pro-inflammatory cytokines while concurrently promoting the release of anti-inflammatory cytokines, as evidenced by various in-vivo, ex-vivo, and in-vitro studies. At a cellular level, macrolides facilitate the phagocytosis of apoptotic cells by alveolar macrophages and mitigate the chemotactic response of neutrophils (Hodge et al. 2006; Hodge et al. 2008). Additionally, they reduce neutrophil degranulation and adhesion and may attenuate the production of reactive oxygen species, thereby dampening inflammatory processes (Postma et al. 2019; Vardakas et al. 2017; Ceccato et al. 2019). Furthermore, macrolides influence adaptive immunity by enhancing apoptosis in T-lymphocytes and exerting a suppressive effect on proinflammatory cytokine production (Williams et al. 2005; Kadota et al. 2005).
Clinical studies in pneumonia have also shown that patients treated with macrolides can undergo alterations in serum concentrations of inflammatory biomarkers. It is hypothesised that sepsis and pneumonia can be characterised by the phenomenon of immunoparalysis, where proinflammatory cytokines such as interleukin(IL)-8 can be reduced in relation to anti-inflammatory cytokines, such as IL-10, with attenuated production of tumour necrosis factor-alpha (TNF-α) (McElvaney et al. 2020). The administration of macrolide antibiotics appears to reverse these ratios. Notably, in the ACCESS trial, patients receiving clarithromycin exhibited lower IL-10 and higher TNF-α levels in peripheral blood mononuclear cells (PMNCs), along with an increased IL-8 to IL-10 ratio on day 4 compared to placebo (Giamarellos-Bourboulis et al. 2024). Collectively, these findings suggest a potential modulation of immune responses and subsequent mitigation of immunoparalysis.
Intra-Class Comparison of Macrolides and Inter-Class Comparison with Fluoroquinolones
The clinical benefits of macrolide antibiotics raise the question of whether the therapeutic potentials are comparable between the agents within this class of medications. Giamarellos-Bourboulis et al. (2024) evaluated clarithromycin versus placebo in patients with pneumonia in RCTs and demonstrated clinical benefits with clarithromycin. However, whether the same benefit can be extrapolated to other macrolides, such as azithromycin, remains unresolved. In a post-hoc analysis of the CAP-START trial, cardiac adverse event rates (defined as new or worsening heart failure, arrhythmia, or myocardial ischaemia) were compared between the beta-lactam monotherapy group and those with macrolide and fluoroquinolone exposure. The results revealed higher event rates in the macrolide group, which was attributed largely to erythromycin and less so to azithromycin and clarithromycin, which may have reflected the larger fluid load needed when using erythromycin (Postma et al. 2019).
The comparability of fluoroquinolones as adjunctive therapy to macrolide antibiotics is also a matter of debate. Current existing data favours the use of macrolides over fluoroquinolones based on prior observational studies, along with systematic reviews and meta-analyses. These sources have consistently demonstrated that macrolide antibiotics are associated with reduced mortality compared to fluoroquinolone use (Vardakas, Trigkidis, and Falagas 2017). In the context of severe CAP, a multicentre observational study focusing on intubated CAP patients with severe sepsis and septic shock found that the addition of macrolides was associated with reduced mortality but not with fluoroquinolone use (Martin-Loeches et al. 2010). These findings highlight the need for further investigation with high-quality RCTs into the relative efficacy of these antibiotic classes.
Macrolides in Specific Populations
As indicated by the currently available studies, there is a preference for macrolide antibiotics, particularly in patients with severe illness. Nevertheless, professional society guidelines currently recommend their use across all severity levels of hospitalised patients. Regarding specific patient phenotypes, those with heightened inflammatory responses may derive greater benefit from macrolide therapy. For instance, a study involving 1715 CAP patients revealed that individuals with elevated C-reactive protein levels experienced lower mortality when treated with beta-lactams in combination with macrolides compared to fluoroquinolone combinations (Ceccato et al. 2019). This highlights the potential for individualised medicine approaches, suggesting that macrolide therapy could be tailored to patients with hyperinflammatory phenotypes rather than employing a uniform treatment strategy.
Role of Corticosteroids
The use of corticosteroids is anchored on the hypothesis that their use as an adjunctive therapy can help mitigate dysregulated immune response that can lead to disproportionate harm in the host (Heming et al. 2018). Their use has remained as a part of sepsis treatment for several decades (Schumer 1976). Previous professional guidelines did not advocate for the routine use of corticosteroids as adjunctive treatment in either CAP or severe CAP, except for refractory septic shock, where their clinical benefit, especially in terms of mortality reduction, remains contested (Martin-Loeches et al. 2023; Metlay et al. 2019). However, the latest Society of Critical Care Medicine (SCCM) guidelines now recommend corticosteroids for severe bacterial CAP (Chaudhuri et al. 9900).
Given the considerable overlap in patient populations, much of our understanding of steroid use in pneumonia can be extrapolated from sepsis studies. Several prominent RCTs have delved into the clinical effects of corticosteroids in this population, yielding conflicting results. The Annane et al. (2002) trial initially showed promising results, with corticosteroid administration reducing 28-day mortality. However, subsequent trials failed to confirm this finding consistently. For instance, the Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock (ADRENAL) trial, evaluating continued hydrocortisone administration, demonstrated reduced mechanical ventilation days in shock patients but found no significant difference in 90-day mortality compared to placebo. Similarly, the earlier Corticosteroid Therapy of Septic Shock (CORTICUS) trial did not reveal a survival benefit, although it was underpowered. Conversely, the Recombinant Human Activated Protein C and Low Dose of Hydrocortisone and Fludrocortisone in Adult Septic Shock (APROCCHSS) trial showed improved overall 90-day survival with hydrocortisone plus fludrocortisone compared to placebo (Annane et al. 2018).
Turning to RCTs specific to pneumonia, one multicentre study by Torres et al. (2015) compared the efficacy of methylprednisolone versus placebo for five days, administered within 36 hours of admission. This trial focused on severe CAP patients, with 70-80% admitted to the ICU, and only included those with high levels of inflammation, as reflected by elevated CRP levels (>150 milligrams per litre) on admission. While the steroid group experienced less late treatment failure, there was no significant difference in mortality. A Cochrane review conducted in 2017 prior to this trial encompassing 17 trials showed a significant reduction in mortality among severe CAP patients receiving corticosteroids compared to placebo (Stern et al. 2017). Another study, conducted in 2022 as a double-blind, randomised, placebo-controlled trial at 42 veterans affairs (VA) medical centres in the United States, enrolled patients meeting specific modified ATS/IDSA severity criteria, admitted to ICU or step-down units (SDU). These patients received a methylprednisolone loading dose of 40 milligrams followed by maintenance infusion for 20 days with tapering, with the primary outcome assessed being all-cause mortality at 60 days. Unfortunately, the study did not show a significant difference in mortality. However, it is important to note that the generalisability of the study may be limited due to the study’s highly specific population and an underpowered design.
Lastly, in a recent RCT, the Community-Acquired Pneumonia: Evaluation of Corticosteroids (CAPE-COD) trial, conducted across multiple centres in France, a significant reduction in 28-day mortality was observed for patients hospitalised with severe CAP who received hydrocortisone compared to placebo. The study consisted of a high proportion of patients on high-flow nasal cannula (HFNC), with only approximately 23% on mechanical ventilation in the treatment arm and 21.5% in the placebo arm. Hydrocortisone administration improved 28-day mortality, and additional findings included a reduced rate of intubation and vasopressor use in the hydrocortisone arm. Notably, the number needed to treat (NNT) to prevent a single death, based on the estimate of the 2017 Cochrane review, was similar, at approximately 18 patients. Furthermore, after the CAPE-COD trial, an additional meta-analysis incorporating both the CAPE-COD trial and the 2022 VA trial found an overall mortality benefit with corticosteroids (Pitre et al. 2023).
Corticosteroids in Specific Populations
While the data on corticosteroid therapy in severe CAP has presented conflicting findings, a growing body of evidence suggests potential improvements in clinical outcomes, but it is unclear if all severe CAP patients should be treated. Subgroup analyses, such as those conducted in the CAPE-COD trial, have indicated that patients with elevated serum CRP (>15 milligrams per decilitre) may derive a more significant survival benefit from corticosteroid therapy compared to those with lower CRP levels. This observation aligns with findings from studies like the one conducted by Torres et al. (2015), which demonstrated a reduced rate of treatment failure in severe CAP patients with a high level of inflammation and suggests that individuals with a hyperinflammatory profile might benefit most from targeted corticosteroid therapy.
It is also important to recognise that corticosteroid therapy carries inherent risks. Corticosteroid therapy is associated with increased mortality related to secondary infection in patients with influenza pneumonia. Prolonged administration has been linked to an increased risk of invasive fungal infections, a concern particularly relevant given the rise in multidrug-resistant species like Candida auris since the COVID-19 pandemic (Biran et al. 2023; Pakdel et al. 2021; Gangneux et al. 2022). Moreover, there is evidence to suggest that certain patient populations, such as those with lymphopenia, may experience harm from steroid administration (Torres et al. 2019). Therefore, while corticosteroids hold promise as a potential adjunctive therapy in severe CAP, careful consideration of their risks and benefits is essential in clinical decision-making.
Conclusion and Recommendations
In summary, the evolving evidence underscores the potential clinical benefits of anti-inflammatory therapeutics in the management of severe community-acquired pneumonia (CAP). Studies, such as the ACCESS trial for macrolide antibiotics and the CAPE-COD trial for corticosteroids, have revealed improvements in various clinical outcomes, including mortality. However, uncertainties persist regarding the optimal timing and patient selection for these treatments, alongside concerns about potential adverse effects. Recognising the importance of precision medicine, there's a growing understanding that patients with a hyperinflammatory profile may derive enhanced benefits from these therapies. This highlights the need for careful patient selection and personalised treatment strategies to maximise efficacy while minimising risks. Moreover, exploring non-antibiotic macrolides could offer an intriguing avenue for future therapeutic interventions. Nevertheless, further large-scale clinical trials are warranted to delineate the precise role of anti-inflammatory therapies in severe CAP management and refine treatment guidelines accordingly.
Conflict of Interest
Dr Di Pan has no conflicts of interest to declare. Dr Michael S Niederman has been a consultant for Pfizer and Merck.
References:
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med. 2013;369:840-51.
Annane D, Renault A, Brun-Buisson C et al. (2018) Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 378:809-18.
Annane D, Sébille V, Charpentier C et al. (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 288:862-71.
Asgrimsson V, Gudjonsson T, Gudmundsson GH, Baldursson O (2006) Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 50:1805-12.
Biran R, Cohen R, Finn T et al. (2023) Nationwide Outbreak of Candida auris Infections Driven by COVID-19 Hospitalizations, Israel, 2021–2022. Emerg Infect Dis. 29:1297.
Cavallazzi R, Furmanek S, Arnold FW et al. (2020) The Burden of Community-Acquired Pneumonia Requiring Admission to ICU in the United States. Chest. 158:1008-16.
Ceccato A, Cilloniz C, Martin-Loeches I et al. (2019) Effect of Combined β-Lactam/Macrolide Therapy on Mortality According to the Microbial Etiology and Inflammatory Status of Patients With Community-Acquired Pneumonia. Chest. 155:795-804.
Chaudhuri D, Nei AM, Rochwerg B et al. (2024) 2024 Focused Update: Guidelines on Use of Corticosteroids in Sepsis, Acute Respiratory Distress Syndrome, and Community-Acquired Pneumonia. Crit Care Med.
Dremsizov T, Clermont G, Kellum JA et al. (2006) Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 129:968-78.
Gangneux JP, Dannaoui E, Fekkar A et al. (2022) Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study. Lancet Respir Med. 10:180-90.
García Vázquez E, Mensa J, Martínez JA et al. (2005)Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis. 24:190-5.
Garin N, Genné D, Carballo S et al. (2014) β-Lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 174:1894-901.
Gattarello S, Borgatta B, Solé-Violán J et al. (2014) Decrease in mortality in severe community-acquired pneumococcal pneumonia: impact of improving antibiotic strategies (2000-2013). Chest. 146:22-31.
Giamarellos-Bourboulis EJ, Siampanos A, Bolanou A et al. (2024) Clarithromycin for early anti-inflammatory responses in community-acquired pneumonia in Greece (ACCESS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med.
GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392(10159):1736-1788.
Heming N, Sivanandamoorthy S, Meng P et al. (2018) Immune Effects of Corticosteroids in Sepsis. Front Immunol. 9:1736.
Hoban DJ, Doern GV, Fluit AC et al. (2001) Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 32 Suppl 2:S81-93.
Hodge S, Hodge G, Brozyna S et al. (2006) Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J. 28:486-95.
Hodge S, Hodge G, Jersmann H et al. (2008) Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 178:139-48.
Ichimiya T, Takeoka K, Hiramatsu K et al. (1996) The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy. 42:186-91.
Ichimiya T, Yamasaki T, Nasu M (1994) In-vitro effects of antimicrobial agents on Pseudomonas aeruginosa biofilm formation. J Antimicrob Chemother. 34:331-41.
Kadota J, Mizunoe S, Kishi K et al. (2005) Antibiotic-induced apoptosis in human activated peripheral lymphocytes. Int J Antimicrob Agents. 25:216-20.
Kelly C, Chalmers JD, Crossingham I et al. (2018) Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Rev. 3:Cd012406.
Lodise TP, Kwa A, Cosler L et al. (2007) Gupta R, Smith RP. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother. 51:3977-82.
Mandell LA, Wunderink RG, Anzueto A et al. (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 44 Suppl 2:S27-72.
Marrie TJ, Shariatzadeh MR (2007) Community-acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore). 86:103-11.
Martin-Loeches I, Lisboa T, Rodriguez A et al. (2010) Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 36:612-20.
Martin-Loeches I, Torres A, Nagavci B et al. (2023) ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 49:615-32.
Martínez JA, Horcajada JP, Almela M et al. (2003) Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 36:389-95.
McElvaney OJ, McEvoy NL, McElvaney OF et al. (2020) Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am J Respir Crit Care Med. 202:812-21.
Metersky ML, Ma A, Houck PM, Bratzler DW (2007) Antibiotics for bacteremic pneumonia: Improved outcomes with macrolides but not fluoroquinolones. Chest. 131:466-73.
Metlay JP, Waterer GW, Long AC et al. (2019) Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 200:e45-e67.
Pakdel F, Ahmadikia K, Salehi M et al. (2021) Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 64:1238-52.
Pitre T, Abdali D, Chaudhuri D et al. (2023) Corticosteroids in Community-Acquired Bacterial Pneumonia: a Systematic Review, Pairwise and Dose-Response Meta-Analysis. J Gen Intern Med. 38:2593-606.
Postma DF, Spitoni C, van Werkhoven CH et al. (2019) Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: post-hoc analysis of a cluster-randomized trial. BMC Infect Dis. 19:17.
Postma DF, van Werkhoven CH, van Elden LJ et al. (2015). Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 372:1312-23.
Restrepo MI, Mortensen EM, Waterer GW et al. (2009) Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J. 33:153-9.
Riley PD, Aronsky D, Dean NC (2004) Validation of the 2001 American Thoracic Society criteria for severe community-acquired pneumonia. Crit Care Med. 32:2398-402.
Schumer W (1976) Steroids in the treatment of clinical septic shock. Ann Surg. 184:333-41.
Sligl WI, Asadi L, Eurich DT et al. (2014) Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 42:420-32.
Song JH, Jung SI, Ko KS et al. (2004) High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 48:2101-7.
Starner TD, Shrout JD, Parsek MR et al. (2008) Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob Agents Chemother. 52:137-45.
Stern A, Skalsky K, Avni T et al. (2017) Corticosteroids for pneumonia. Cochrane Database Syst Rev. 12:Cd007720.
Tagaya E, Tamaoki J, Kondo M, Nagai A (2002) Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest. 122:213-8.
Torres A, Sibila O, Ferrer M et al. (2015) Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 313:677-86.
Torres A, Ceccato A, Ferrer M et al. (2019) Effect of Corticosteroids on C-Reactive Protein in Patients with Severe Community-Acquired Pneumonia and High Inflammatory Response: The Effect of Lymphopenia. J Clin Med. 8:1461.
Vardakas KZ, Trigkidis KK, Falagas ME (2017) Fluoroquinolones or macrolides in combination with β-lactams in adult patients hospitalized with community acquired pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect. 23:234-41.
Williams AC, Galley HF, Watt AM, Webster NR (2005) Differential effects of three antibiotics on T helper cell cytokine expression. J Antimicrob Chemother. 56:502-6.
Wozniak DJ, Keyser R (2004) Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest. 125:62S-69S; quiz 69S.
Yamaya M, Azuma A, Takizawa H et al. (2012) Macrolide effects on the prevention of COPD exacerbations. Eur Respir J. 40:485-94.
Yasuda H, Ajiki Y, Koga T, Yokota T (1994) Interaction between clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrob Agents Chemother. 38:138-41.