ICU Management & Practice, Volume 23 - Issue 5, 2023
Subarachnoid haemorrhage (SAH) carries a high disease-specific burden. Epidemiological studies have observed a reduction in the incidence of SAH from 1990 to 2019. However, the same trend was not observed for intrahospital mortality. Several studies focusing on the evaluation of SAH management across the world have shown a high heterogeneity in care standards, with potential implications on the prognosis. Short-term and long-term outcomes need to be analysed. Besides motor disability, neurocognitive sequels are common and deeply affect the quality of life of a person.
Introduction
Acute subarachnoid haemorrhage (SAH) represents a life-threatening condition characterised by a hyperacute accumulation of blood in the subarachnoid space. Generally, blood spreads peripherally to the cerebral convexities in a diffuse manner. However, radiological evaluation of blood distribution can show a predominance of subarachnoid blood in the perimesencephalic and basal Sylvian cisterns (Perimesencephalic SAH) or in isolated cerebral convexity (Marder et al. 2014). Furthermore, secondary intraventricular haemorrhage can accompany SAH with possible implications on the prognosis (Darkwah et al. 2018; Zanaty et al. 2018).
The main cause of SAH is due to the rupture of a cerebral aneurysm. However, in up to 15% of the cases, initial neuroimaging evaluation (i.e., angiography) is not able to identify an aneurysm; condition defined as sine materia or subarachnoid haemorrhage of unknown origin (Bacigaluppi et al. 2022; Mohan and Tauseen 2020). Hyperacute accumulation of blood in the subarachnoid space can also represent a radiological feature observed in several diseases that should be considered as differential diagnosis (e.g., traumatic subarachnoid haemorrhage, arteriovenous malformations ruptures, intraparenchymal haemorrhage, dural fistula ruptures, cerebral venous infarction) (Oppenheim et al. 2005; Rasyid et al. 2022; Arévalo-Lorido and Carretero-Gómez 2015). Indeed, SAH, mostly secondary to the rupture of a cerebral aneurysm, is characterised by different and typical clinical history (Claassen and Park 2022).
According to 2020 World Health Organization (WHO) estimates, stroke, also referred to as an acute cerebrovascular accident, represents the second cause of mortality in the world, with 11% of cases, followed only by cardiovascular disease with 16% of deaths (Feigin et al. 2022; James et al. 2019. Worldwide, stroke represents the second cause of years lost to premature death and life lived with disability (Vos et al. 2020). SAH represents the third most frequent cause of stroke and although it represents only 5% of all strokes, SAH is characterised by some aspects that make it an important pathology to study. SAH carries a high disease-specific burden (Krishnamurthi et al. 2020). Considering the health, social, and economic factors that determine the cost that SAH and the consequent disability have on the individual and on society, SAH results in high direct and indirect costs (English 2020). One-quarter of SAH patients (around 22-26%) die before arriving at the hospital, and 50% of survivors have various degrees of long-term disability (van Gijn and Rinkel 2001). This aspect is even more alarming if we consider that they are generally healthy patients in an economically active phase of life with a long life expectancy, leading to many years of reduced quality of life (English 2020). The specific burden of a disease can be calculated with disability adjusted life year (DALY), the sum of the years of life lost (YLL), and the years lived with disability (YLD) (Martinez et al. 2019; Rivero-Arias et al. 2010). In this paper, we aim to outline an epidemiological perspective on SAH.
Epidemiology of SAH
The crude incidence of SAH in the world is approximately 7.9 per 100,000 people/year (Etiman et al. 2019). There is significant variability in the incidence of SAH around the world, with the highest incidence in Finland and Japan (Figure 1) (Hughes et al. 2018). A 2019 study of the Global Burden of Disease (GBD) estimated that there were 1,018,000 cases of SAH in the world, with a reduction in age-standardised rates from 1990 to 2019 (Figure 2) (James et al. 2019). The reduction in incidence seems to be attributable, above all, to a better control of blood pressure values and a reduction in smoking habits. Linear regression analyses showed that for every mmHg reduction in systolic blood pressure, the SAH incidence tends to reduce by 7.1%. Furthermore, every percentage reduction in smoking habits translates into a reduction of 2.4% in SAH incidence (Etiman et al. 2019). Remarkably, in the study of GBD, high systolic blood pressure was identified as the most important risk factor for SAH ((Feigin et al. 2021). World Bank low-income and upper-middle-income countries showed a lower percentage of SAH incidence in comparison with the high-income countries (7.9% versus 19.7%) (James et al. 2019). It can be speculated that prevention campaigns have played a vital part in the global reduction of SAH. Even more, against the global tendency, an increase in incidence over time has been observed in countries such as China in middle-aged people (Darkwah et al. 2018; Vos et al. 2020). This trend has been explained mainly by the changes in lifestyles in an increasingly industrialised country (Zhang et al. 2013).
Unfortunately, epidemiological studies have not observed the same declining trend in mortality. In-hospital mortality for SAH has not changed in the last 20 years (around 13%) (Korja et al. 2016; Wahood et al. 2022). Consequently, it is necessary to focus our attention on improving our standards of care after the bleeding. Therefore, the prognosis of the patient is enhanced by correct health management based on a deeper knowledge of the physiopathological aspects of the disease. However, it is important to highlight that to better characterise a disease, it is not enough to analyse the outcome in terms of mortality or DALY (Robba et al. 2020; Herridge and Azoulay 2023). It is necessary to develop a deeper and more detailed analysis of the complications that may occur, both during hospital stay and at discharge, to fully understand the pathophysiology of SAH and to have important feedback on our standard of care.
A further element of interest is represented by the management of the patient with an unruptured cerebral aneurysm, which generally represents incidental imaging findings on neuroimaging. It is estimated that approximately 15 million people in Europe have an unruptured aneurysm with a prevalence of 3.2% of the population (Vlka et al. 2011; Wagner and Stenger 2005). A critical element is represented by the fact that there is no unanimity on the management of unruptured aneurysms, as the risk and benefit of a neurosurgical or neuroradiological procedure must be assessed. The current literature is not conclusive regarding the possible correlation between the treatment of unruptured cerebral aneurysm and the risk of SAH (Tsutsumi et al. 1999; Etminan et al. 2022; Raymond et al. 2008). Nowadays, the decision to treat a non- ruptured aneurysm requires knowledge of its natural history and the evaluation of the risk of rupture since prophylactic treatment of intracranial aneurysm is burdened by a risk of mortality and treatment-related morbidity of up to 5% (Pontes et al. 2021). Several rupture prediction tools for asymptomatic intracranial aneurysm (i.e. UIATS, PHASES, ELAPSS scores) were developed to aid the clinician in the evaluation of the possible natural history of aneurysm rupture on the basis of aneurysm-and-patients’ characteristics (i.e. hypertension, age, size, aneurysm in posterior circulation and irregularities in shape) (Greving et al. 2014; Bijlenga et al. 2017; Backes et al. 2017). Unfortunately, the current literature led to conflicting results on the correlation between these scores and the risk of rupture (Feng et al. 2021; Hernández-Durán et al. 2021).
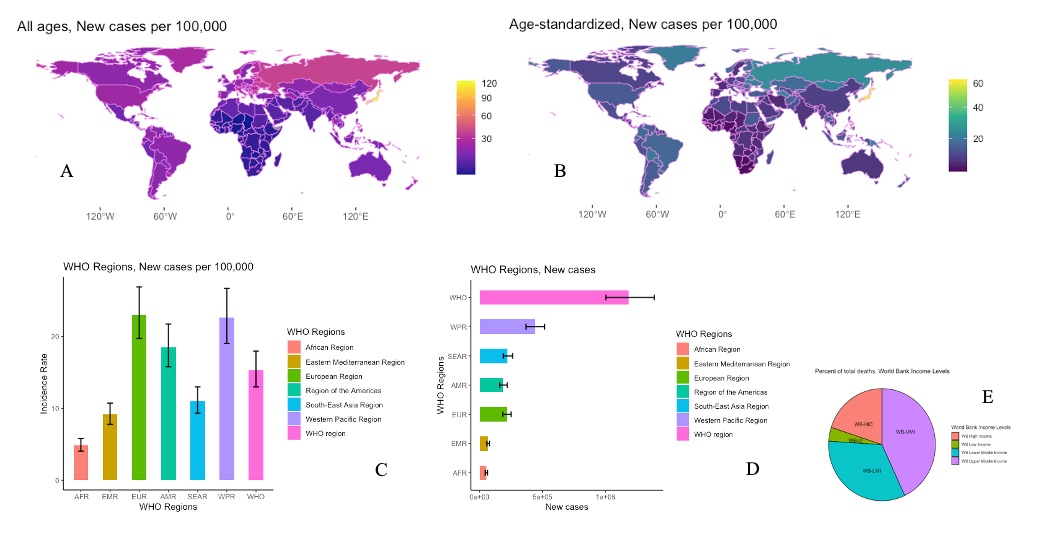
Figure 1. Epidemiological incidence and geographical distribution of SAH according to Global Burden Disease. A) World Map, Geographical distribution of SAH: Incidence Rate, New cases per 100,000, both sexes, All ages, 2019; B) World Map, Geographical distribution of SAH: Incidence Rate, New cases per 100,000, both sexes, Age-standardised, 2019; C) Bar Graph showing the new cases per 100,00 of SAH by WHO Regions, both sexes, All ages, 2019; D) Bar Graph showing the estimated new cases of SAH by WHO Regions, both sexes, All ages, 2019; E)Pie Chart showing percentage of deaths by World Bank Income Levels, both sexes, All ages, 2019. Source: Institute for Health Metrics and Evaluation. Used with permission. All rights reserved. Data were extracted at https://vizhub.healthdata.org/ gbd-results/ accessed on the 26th of October 2023. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME), 2020(12), https://doi.org/10.6069/1D4Y-YQ37. All analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021). Bar graph and Pie chart were obtained via the ggplot2 R package (v3.4.4; Wickham 2016). Bar errors indicating 95% Uncertainty Intervals. World Maps was obtained via the ggplot2 R package (v3.4.4; Wickham 2016), the tidyverse R package (v2.0.0; Wickham et al. 2019), rnaturalearth R package (v3.4; Massicotte and South 2023). SAH: Subarachnoid Haemorrhage; AFR: African Region; EMR: Eastern Mediterranean Region; EUR: European Region; AMR: Region of the Americas; SEAR: South-East Asian Region; WPR: Western Pacific Region; WHO: World Health Organisation; WB-HIC: World Bank High Income; WB-LI: World Bank Low Income; WB-LMI: World Bank Lower Middle Income; WB-UMI: World Bank Upper Middle Income.
Practice Variability
The 2023 guidelines of the American Heart Association/American Stroke Association emphasised the need to manage SAH patients throughout their entire care journey (Hoh et al. 2023). Furthermore, these guidelines highlighted some critical elements as having a decisive impact on the outcome:
- System of care characteristics (i.e. high-volume centre with a dedicated neurointensive care unit)
- Prevention of aneurismal rebleeding
- Early detection and management of medical complications associated with SAH (i.e. early brain injury and delayed cerebral ischaemia [DCI])
Professor Claassen's seminar underlined that managing SAH patients starts before reaching the hospital by selecting a high-volume centre (Claassen and Park 2022). Healthcare centralisation plays a critical role in patients’ management of the condition. To provide the best care for patients quickly, it is necessary to distribute specialised facilities evenly throughout the country. Several studies have already observed the correlation between the choice of high-volume centre and outcome (McNeill et al. 2013; Lindgren et al. 2019; Rush et al. 2017). Moreover, rebleeding and DCI are associated with worse mortality and morbidity. Consequently, minimising rebleeding and the prevention and early detection of DCI represent important goals in SAH management (Figure 3) (Huenges Wajer et al. 2019; Duan et al. 2018).
Unfortunately, several studies focusing on the evaluation of the management of SAH have shown a high heterogeneity in the management of SAH across the world. In Winkel's survey, including 230 centres around the world, the authors observed a high variability in the timing of treatment and in the type of treatment for aneurysm repair between centres (de Winkel et al. 2021). This survey found a statistically significant difference between Europe and the USA regarding the type of treatment and timing compared to other centres in the world. In the U.S. and Europe, aneurysms were generally treated with endovascular surgery (72% and 705 vs. 51% globally) and generally within 24 h (77% and 64% vs 46%). In 2015, the European Society of Anaesthesiology published a survey on the European practice of SAH management in 268 centres (Velly et al. 2015). Statistically significant differences were observed in the ICU admission criteria, in the treatment of aneurysm and in the management of vasospasm and DCI. This heterogeneity was also confirmed in a study conducted in 22 Italian neurosurgical centres (Citerio et al. 2007). This phenomenon may be explained mainly by the fact that little evidence is available, and practice differences may be based on local policies rather than on solid scientific data.
Remarkably, in 2019, Dijkland et al. (2019) observed a difference in clinical outcome (i.e. GOS and mRS at 3 months) among different medical centres and different countries after SAH. The authors analysed data from 5972 patients with SAH from the SAHIT repository, including 179 centres (i.e., 20 countries). They applied logistic regression to account for a patient’s characteristic (i.e. age, hypertension, WFNS, Fisher grade, aneurysm location) and timing of treatment. Consequently, the difference in outcomes observed could not be justified by these potential confounding factors. Accordingly, further studies must be warranted on the correlation between treatment heterogeneity and outcome.
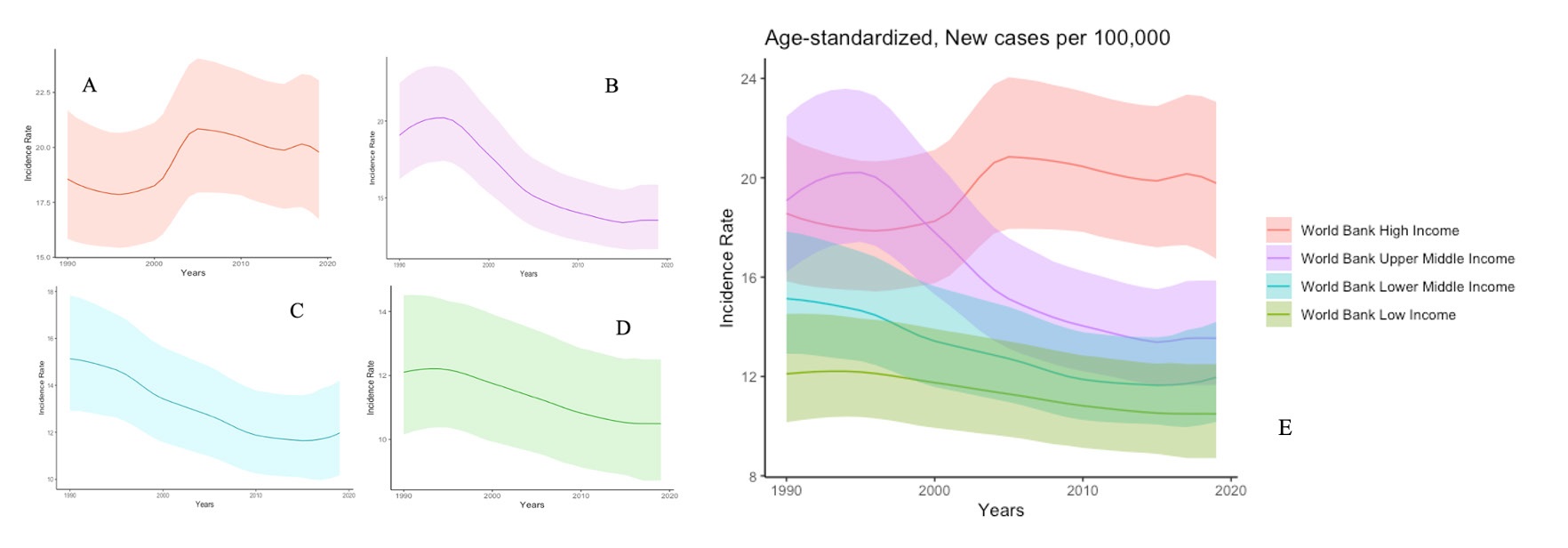
Figure 2. Trends in Incidence Rate of SAH according to Global Burden Disease. A) Trend Chart of SAH: Incidence Rate, New cases per 100,000, both sexes, Age-standardised, 1990-2020, World Bank High Income Country; B) Trend Chart of SAH: Incidence Rate, New cases per 100,000, both sexes, Age-standardised, 1990-2020, World Bank Upper Middle Income Country; C) Trend Chart of SAH: Incidence Rate, New cases per 100,000, both sexes, Age-standardised, 1990-2020, World Bank Lower Middle Income Country; D) Trend Chart of SAH: Incidence Rate, New cases per 100,000, both sexes, Age-standardised, 1990-2020, World Bank Low Income Country; E) Trend Chart showing Incidence Rate according to the four World Bank Income Level from 1990-2020. Source: Institute for Health Metrics and Evaluation. Used with permission. All rights reserved. Data were extracted at https://vizhub.healthdata.org/gbd-results/ accessed on the 27th of October 2023. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME), 2020(12), https://doi.org/10.6069/1D4Y-YQ37. All analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021). Trend charts showing 95% Uncertainty Intervals. Trend charts were obtained via the ggplot2 R package (v3.4.4; Wickham 2016). SAH: Subarachnoid Haemorrhage.
Outcome
Patients who survive a critical illness and leave the hospital can develop a condition known as post-intensive care syndrome (PICS). PICS affects the physical, mental and emotional aspects of the patients. After ICU discharge, patients may present cognition difficulties and mental health alterations with important impacts on the patients’ and familial quality of life. Consequently, the detailed evaluation of all the possible complications at discharge allows practitioners to analyse the pathology itself from two points of view:
- to better understand the pathophysiology and improve in-hospital management;
- to outline the best rehabilitation trajectories.
From this perspective, there is a necessity to analyse both short-term and long-term outcomes. After SAH, it is estimated a readmission rate of around 8-10% at 30 days, 16% at 60 days, and 16-26% at 90 days (Rumalla et al. 2018; Dasenbrock et al. 2017; Liang et al. 2018). Of note, it has also been estimated that 14% of readmissions are potentially preventable (49). The analysis of the causes of readmission allows us to identify both modifiable and non-modifiable risk factors in order to plan preventive interventions for different patient subgroups.
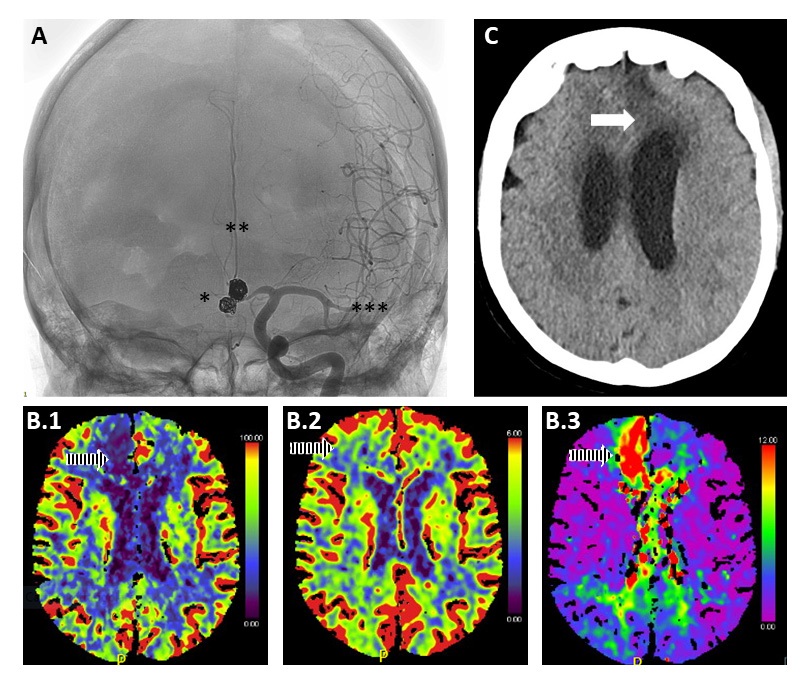
Figure 3. Integration of different imaging modalities in managing patients with SAH with vasospasm. A) Use of angiography to diagnose vasospasm (gold standard). In the image, an asterisk indicates the outcome of aneurysm coiling, two asterisks the presence of severe vasospasm of the anterior cerebral arteries (more remarkable on the right side), and three asterisks diffuse vasospasm of the left middle cerebral artery. B) Use of CT perfusion for the diagnosis of vasospasm. Reduced cerebral blood flow (CBF) is observed (B.1) in the cerebral arteries (more significant on the right side) in the presence of preserved cerebral blood volume (CBV) (B.2), although unable to provide adequate compensation, and (B.3) an increased Tmax due to increased macrovascular resistance. C) Use CT to assess secondary damage development due to DCI/vasospasm. The arrow shows the presence of an infarct-like brain lesion in the territory of the left anterior cerebral artery. In this specific case, aggressive medical and neuroradiological treatment has prevented the development of bilateral frontal ischemic injury.
Regarding long-term outcomes, different kinds of disability can be observed in SAH patients at discharge. Indeed, besides motor disability, neurocognitive sequels are common and deeply affect the quality of life of a person (Nwafor et al. 2023). Up to 47% of post-SAH patients have mood disorders (i.e., depression and/or anxiety) during the first year (Catapano et al. 2023; Kreiter et al. 2013; Ackermark et al. 2017). Even more, personality changes range from 32 to 59%, and post-traumatic stress disorders occurred in around 40% of the patients (Tang et al. 2022; Hedlund et al. 2011). Alongside the quality of life, neurocognitive impairment affects the return to work, resuming of social relationships, and functional outcome (Buunk et al. 2019; Kauranen et al. 2013; Saar et al. 2023). Consequently, the creation of suitable diagnostic, therapeutic and rehabilitation pathways to ensure continuity of care, as well as the best type of rehabilitation that these persons need, represent a further central element to evaluate in order to improve SAH management.
Another key aspect to evaluate is represented by the perception of the patient on his/her own abilities; i.e. not only an objective evaluation of residual functional capacity, but also the individual's own evaluation of his/her performance status. Increasing evidence has shown that a poor grade SAH is not necessarily linked to a low quality of life (Goldberg et al. 2023). Therefore, a discrepancy between disability and the patient's perception of her health and functionality is noticed. In 1983, the sociologist Sol Levine introduced the concept of the "disability paradox" and raised the need to apply the evaluation of the quality of life to disability, moving from an objective to a subjective evaluation of disability (Levine et al. 1981). Indeed, the disability paradox refers to the discrepancy between the patients' perceptions of well-being and their disability level, evaluated objectively.
Neurorehabilitation plays a central supportive role in all of these aforementioned aspects. In 2023, Lindner et al. (2023) observed functional improvement in 57% of patients in the first 3 months and a further 16% improvement at 12 months. These findings outline that improvement was observed, especially during the first months. Indeed, rehabilitation must be seen as a continuum from hospital stay to patient’s home. During hospital stay, it is preferable that rehabilitation programmes start as soon as possible, with a multidisciplinary team drawing a tailored rehabilitation pathway for the convalesce phases (Yataco et al. 2019). Indeed, there must be a transition from the hospital phase to the post-discharge phase that is already planned in the hospital. Unfortunately, there is a huge discrepancy in accessibility to rehabilitation programmes from region to region, leading to health inequality (Strickland et al. 2020).
It is important to outline that the evaluation of outcomes requires the implementation of a follow-up programme for the intensive care unit survivor. The realisation of this programme has to deal with two major issues:
- Organisation, costs and workload of follow-up programmes
- Use a standardised scale for outcome evaluation
It is noteworthy to highlight that the scales used to assess post-SAH functional outcomes were originally created for different neurological conditions (Pace et al. 2018). Consequently, there is a huge need for standardisation of prognostic evaluation using a well-validated scale at a specific time after discharge.
Conclusion
SAH predominantly affects young and healthy populations. SAH is associated with significant premature mortality and disability requiring protracted hospitalisation stays and specific rehabilitation pathways. Reduction in incident rates in the last 20 years could reflect the positive effects of prevention campaigns. Unfortunately, the same trend was not observed for intrahospital mortality. There is a wide variation in how SAH patients are managed globally, which could impact their prognosis. These findings have to be analysed, taking into account the huge heterogeneity of the availability of high-specific competency between countries and centres (i.e., neurocritical care, neurosurgery, neuroradiology, neurorehabilitation). From this perspective, comparative effectiveness research is to be encouraged, focusing on the correlation between practice variabilities and outcomes in order to improve our delivery of care.
Conflict of Interest
None.
Permission - Tables/Figure
Institute for Health Metrics and Evaluation. Used with permission. All rights reserved.
Abbreviations
WHO: World Health Organization
SAH: Subarachnoid Haemorrhage
DALY: Disability Adjusted Life Year
YLL: Years of Life Lost
YLD: Years Lived with Disability
GBD: Global Burden of Disease
UIATS: Unruptured Intracranial Aneurysm Treatment Score
PHASES: Population, Hypertension, Age, Size, Earlier Subarachnoid Haemorrhage, and Site
ELAPSS: Earlier Subarachnoid Haemorrhage, Location of the Aneurysm, Age >60 years, Population, Size of the Aneurysm, and Shape of the Aneurysm
DCI: Delayed Cerebral Ischaemia
GOS: Glasgow Outcome Scale
mRS: modified Rankin Scale
SAHIT: Subarachnoid Haemorrhage International Trialists
WFNS: World Federation of Neurosurgeons
PICS: Post-intensive Care Syndrome
ICU: Intensive Care Unit
References:
Ackermark PY, Schepers VP, Post MW et al. (2017) Longitudinal course of depressive symptoms and anxiety after aneurysmal subarachnoid hemorrhage. Eur J Phys Rehabil Med. 53(1):98-104.
Arévalo-Lorido JC, Carretero-Gómez J (2015)
Cerebral Venous Thrombosis with Subarachnoid Hemorrhage: a Case Report. Clin
Med Res. 13(1):40-3.
Bacigaluppi S, Bragazzi NL, Ivaldi F et al. (2022) Systemic Inflammatory Response in Spontaneous Subarachnoid Hemorrhage from Aneurysmal Rupture versus Subarachnoid Hemorrhage of Unknown Origin. J Inflamm Res. 15:6329-42.
Backes D, Rinkel GJE, Greving JP et al. (2017) ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 88(17):1600-6.
Bijlenga P, Gondar R, Schilling S et al. (2017) PHASES Score for the Management of Intracranial Aneurysm: A Cross-Sectional Population-Based Retrospective Study. Stroke. 48(8):2105-12.
Buunk AM, Spikman JM, Metzemaekers JDM et al. (2019) Return to work after subarachnoid hemorrhage: The influence of cognitive deficits. PLoS One. 14(8):e0220972.
Catapano JS, Rumalla K, Koester SW et al. (2023) Incidence and Prediction of Chronic Depression Following Aneurysmal Subarachnoid Hemorrhage: A Single-Center 17-Year Experience. World Neurosurg. 171:e206-e12.
Citerio G, Gaini SM, Tomei G, Stocchetti N (2007) Management of 350 aneurysmal subarachnoid hemorrhages in 22 Italian neurosurgical centers. Intensive Care Med. 33(9):1580-6.
Claassen J, Park S (2022) Spontaneous subarachnoid haemorrhage. Lancet. 400(10355):846-62.
Darkwah Oppong M, Gembruch O, Herten A et al. (2018) Intraventricular Hemorrhage Caused by Subarachnoid Hemorrhage: Does the Severity Matter? World Neurosurg. 111:e693-e702.
Dasenbrock HH, Angriman F, Smith TR et al. (2017) Readmission After Aneurysmal Subarachnoid Hemorrhage: A Nationwide Readmission Database Analysis. Stroke. 48(9):2383-90.
de Winkel J, van der Jagt M, Lingsma HF et al. (2021) International Practice Variability in Treatment of Aneurysmal Subarachnoid Hemorrhage. J Clin Med. 10(4).
Dijkland SA, Jaja BNR, van der Jagt M et al. (2019) Between-center and between-country differences in outcome after aneurysmal subarachnoid hemorrhage in the Subarachnoid Hemorrhage International Trialists (SAHIT) repository. J Neurosurg. 1-9.
Duan W, Pan Y, Wang C et al. (2018) Risk Factors and Clinical Impact of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: Analysis from the China National Stroke Registry. Neuroepidemiology. 50(3-4):128-36.
English SW (2020) Long-Term Outcome and Economic Burden of Aneurysmal Subarachnoid Hemorrhage: Are We Only Seeing the Tip of the Iceberg? Neurocrit Care. 33:37-8.
Etminan N, Chang HS, Hackenberg K et al. (2019) Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 76(5):588-97.
Etminan N, de Sousa DA, Tiseo C et al. (2022). European Stroke Organisation (ESO) guidelines on management of unruptured intracranial aneurysms. Eur Stroke J. 7(3):V.
Feigin VL, Brainin M, Norrving B et al. (2022) World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke 17(1):18-29.
Feigin VL, Stark BA, Johnson CO et al. (2021) Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20(10):795-820.
Feng X, Tong X, Chen J et al. (2021) External Validation of the PHASES Score in Patients with Multiple Intracranial Aneurysms. J Stroke Cerebrovasc Dis. 30(5):105643.
Goldberg J, Z'Graggen WJ, Hlavica M et al. (2023) Quality of Life After Poor-Grade Aneurysmal Subarachnoid Hemorrhage. Neurosurgery. 92(5):1052-7.
Greving JP, Wermer MJ, Brown RD Jr et al. (2014) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 13(1):59-66.
Hedlund M, Zetterling M, Ronne-Engström E et al. (2011) Depression and post-traumatic stress disorder after aneurysmal subarachnoid haemorrhage in relation to lifetime psychiatric morbidity. Br J Neurosurg. 25(6):693-700.
Hernández-Durán S, Mielke D, Rohde V, Malinova V (2021) Is the unruptured intracranial aneurysm treatment score (UIATS) sensitive enough to detect aneurysms at risk of rupture? Neurosurg Rev. 44(2):987-93.
Herridge MS, Azoulay É (2023) Outcomes after Critical Illness. N Engl J Med. 388(10):913-24.
Hoh BL, Ko NU, Amin-Hanjani S et al. (2023) 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 54(7):e314-e70.
Hughes JD, Bond KM, Mekary RA et al. (2018) Estimating the Global Incidence of Aneurysmal Subarachnoid Hemorrhage: A Systematic Review for Central Nervous System Vascular Lesions and Meta-Analysis.
Huenges Wajer IMC, Hendriks ME, Witkamp TD et al. (2019) The relationship between ischaemic brain lesions and cognitive outcome after aneurysmal subarachnoid haemorrhage. J Neurol. 266(9):2252-7.
James SL, Theadom A, Ellenbogen RG et al. (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18(1):56-87.
Kauranen T, Turunen K, Laari S (2013) The severity of cognitive deficits predicts return to work after a first-ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 84(3):316-21.
Korja M, Lehto H, Juvela S, Kaprio J (2016) Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 87(11):1118-23.
Kreiter KT, Rosengart AJ, Claassen J et al. (2013) Depressed mood and quality of life after subarachnoid hemorrhage. J Neurol Sci. 335(1-2):64-71.
Krishnamurthi RV, Ikeda T, Feigin VL et al. (2020) Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology. 54(2):171-9.
Levine S, Elinson J, Feldman J (1981). Does medical care do any good?
Liang JW, Cifrese L, Ostojic LV et al. (2018) Preventable Readmissions and Predictors of Readmission After Subarachnoid Hemorrhage. Neurocrit Care. 29(3):336-43.
Lindgren A, Burt S, Bragan Turner E et al. (2019) Hospital case-volume is associated with case-fatality after aneurysmal subarachnoid hemorrhage. Int J Stroke. 14(3):282-9.
Lindner A, Brunelli L, Rass V et al. (2023) Long-Term Clinical Trajectory of Patients with Subarachnoid Hemorrhage: Linking Acute Care and Neurorehabilitation. Neurocrit Care. 38(1):138-48.
Marder CP, Narla V, Fink JR, Tozer Fink KR (2014) Subarachnoid hemorrhage: beyond aneurysms. AJR Am J Roentgenol 202(1):25-37.
Martinez R, Soliz P, Caixeta R, Ordunez P (2019) Reflection on modern methods: years of life lost due to premature mortality—a versatile and comprehensive measure for monitoring non-communicable disease mortality. Int J Epidemiol. 48(4):1367-76.
McNeill L, English SW, Borg N et al. (2013) Effects of institutional caseload of subarachnoid hemorrhage on mortality: a secondary analysis of administrative data. Stroke. 44(3):647-52.
Mohan V, Tauseen RA (2020) Spontaneous pneumomediastinum in COVID-19. BMJ Case Rep. 132020.
Nwafor DC, Kirby BD, Ralston JD et al. (2023) Neurocognitive Sequelae and Rehabilitation after Subarachnoid Hemorrhage: Optimizing Outcomes. J Vasc Dis. 2(2):197-211.
Oppenheim C, Domigo V, Gauvrit JY et al. (2005) Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol. 26(3):614-7.
Pace A, Mitchell S, Casselden E et al. (2018) A subarachnoid haemorrhage-specific outcome tool. Brain. 141(4):1111-21.
Pontes FB, da Silva EM, Baptista-Silva JC (2021) Vasconcelos V. Treatments for unruptured intracranial aneurysms. Cochrane Database Syst Rev. (5).
Rasyid A, Mesiano T, Kurniawan M et al. (2022) Spontaneous subarachnoid hemorrhage due to arteriovenous malformation mimicking migraine: A case report. Radiol Case Rep. 17(3):790-3.
Raymond J, Guillemin F, Proust F et al. (2008) Unruptured Intracranial Aneurysms: A Critical Review of the International Study of Unruptured Intracranial Aneurysms (ISUIA) and of Appropriate Methods to Address the Clinical Problem. Interv Neuroradiol. 14(1):85-96.
Rivero-Arias O, Gray A, Wolstenholme J et al. (2010) Burden of disease and costs of aneurysmal subarachnoid haemorrhage (aSAH) in the United Kingdom. Cost Eff Resour Alloc. 8:6.
Robba C, Sonneville R, Meyfroidt G (2020) Focus on neuro-critical care: combined interventions to improve relevant outcomes. Intensive Care Med. 46:1027-9.
Rumalla K, Smith KA, Arnold PM, Mittal MK (2018) Subarachnoid Hemorrhage and Readmissions: National Rates, Causes, Risk Factors, and Outcomes in 16,001 Hospitalized Patients. World Neurosurg. 110:e100-e11.
Rush B, Romano K, Ashkanani M et al. (2017) Impact of hospital case-volume on subarachnoid hemorrhage outcomes: A nationwide analysis adjusting for hemorrhage severity. J Crit Care. 37:240-3.
Saar K, Tolvanen A, Poutiainen E, Aro T (2023) Returning to Work after Stroke: Associations with Cognitive Performance, Motivation, Perceived Working Ability and Barriers. J Rehabil Med. 55:jrm00365.
Strickland BA, Mert M, Ravina K et al. (2020) Discrepancy in Neurologic Outcomes Following Aneurysmal Subarachnoid Hemorrhage as a Function of Socioeconomic Class. World Neurosurg. 138:e787-e94.
Tang WK, Wang L, Tsoi KKF et al. (2022) Personality changes after subarachnoid hemorrhage: A systematic review and meta-analysis. J Psychosom Res. 156:110762.
Tsutsumi K, Ueki K, Usui M et al. (1999) Risk of subarachnoid hemorrhage after surgical treatment of unruptured cerebral aneurysms. Stroke. 30(6):1181-4.
van Gijn J, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 124(Pt 2):249-78.
Velly LJ, Bilotta F, Fàbregas N et al. (2015) Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol. 32(3):168-76.
Vlak MH, Algra A, Brandenburg R, Rinkel GJ (2011) Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 10(7):626-36.
Vos T, Lim SS, Afshin A et al. (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396(10258):1204-22.
Wahood W, Rizvi AA, Alexander AY et al. (2022) Trends in Admissions and Outcomes for Treatment of Aneurysmal Subarachnoid Hemorrhage in the United States. Neurocrit Care. 37(1):209-18.
Wagner M, Stenger K (2005) Unruptured intracranial aneurysms: using evidence and outcomes to guide patient teaching. Crit Care Nurs Q. 28(4):341-54.
Yataco RA, Arnold SM, Brown SM et al. (2019) Early Progressive Mobilization of Patients with External Ventricular Drains: Safety and Feasibility. Neurocrit Care. 30(2):414-20.
Zanaty M, Nakagawa D, Starke RM et al. (2018) Intraventricular extension of an aneurysmal subarachnoid hemorrhage is an independent predictor of a worse functional outcome. Clin Neurol Neurosurg. 170:67-72.
Zhang J, Liu G, Arima H et al. (2013) Incidence and risks of subarachnoid hemorrhage in China. Stroke. 44(10):2891-3.






















