Intrahepatic cholangiocarcinoma (ICC) is the second most common liver cancer after hepatocellular carcinoma, with rising global incidence and mortality rates. Surgical resection is a curative approach for ICC. However, due to non-specific symptoms, 70% of patients are diagnosed at advanced stages with unresectable tumours, leading to a poor prognosis and high recurrence risk post-operation. Current prognosis predictions rely on conventional factors like tumour stage and lymph node status, which often fall short in accuracy. Tumour-associated tertiary lymphoid structures (TLSs), immune cell aggregates resembling secondary lymphoid organs, have gained attention for their potential prognostic value and implications for immunotherapy. A recent study published in Insights into Imaging aimed to use radiomic signature and CT imaging features to predict intra-tumour TLSs status preoperatively and assess its correlation with survival in ICC patients.
The Role of Tumour-Associated Tertiary Lymphoid Structures in ICC Prognosis
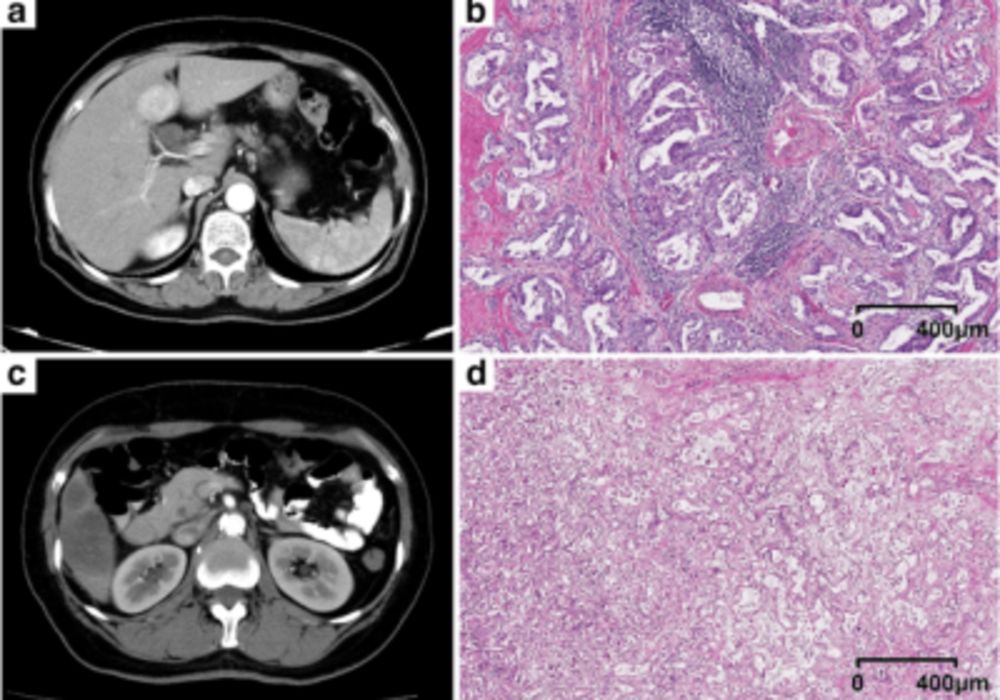
The poor prognosis in cholangiocarcinoma has been linked to features at the cellular and molecular levels, notably a highly desmoplastic tumour microenvironment (TME) with intense immune and stromal cell infiltration. Tumour-associated tertiary lymphoid structures (TLSs), immune cell aggregates resembling secondary lymphoid organs, have gained attention for their potential prognostic value and implications for immunotherapy. Some studies found that intra-tumour TLSs correlate with better survival outcomes. Given that TLSs in ICC can only be confirmed through pathology, preoperative prediction of TLSs status, especially for unresectable cases, is crucial. Radiomics, which analyses quantitative information from images to reflect tumour characteristics and heterogeneity, can aid in this prediction. Computed tomography (CT) is essential for non-invasively diagnosing and managing ICC.
Retrospective Analysis of CT Scans in Surgically Confirmed ICC Patients
This retrospective study analysed preoperative CT scans of 333 patients with surgically confirmed cholangiocarcinoma between November 2010 and August 2020 from two medical centres. The training cohort from centre 1 consisted of 226 patients, while the external validation cohort from centre 2 comprised 107 patients, spanning from May 2015 to November 2019. Inclusion criteria were: patients with confirmed ICC by histology, those with preoperative liver dynamic contrast-enhanced (CE) CT data within a month before surgery, R0 resection performed with postoperative pathological specimens to identify TLSs, and patients without prior treatment for ICC. Exclusion criteria encompassed patients with hilar or extrahepatic cholangiocarcinoma, mixed primary liver cancer types, missing or non-contrast preoperative liver CT data, and previous treatments like chemotherapy, radiotherapy, or radiofrequency ablation. Ultimately, 116 patients (86 from the training cohort and 30 from the external validation cohort) met the study's criteria across the two medical centres.
Predictive Value of TLSs and Radiomics in Preoperative CT Scans
This study revealed that intra-tumoral tertiary lymphoid structures (TLSs) serve as an effective predictor for a favourable prognosis in intrahepatic cholangiocarcinoma (ICC), aligning with prior research. TLSs also indicate a positive response to immunotherapy across various solid tumours. The study aimed to predict TLSs status preoperatively using clinical and radiomics models. The combined model, integrating both, had the best prediction performance, outperforming individual models. While the significance of intra-tumoral TLSs is recognised, no prior research explored correlations between imaging features and TLSs status. This study found that TLSs-positive ICC more frequently exhibited arterial diffuse hyperenhancement on imaging, possibly due to increased cellular areas with less fibrosis. The composition of TLSs consists of various immune infiltrates, potentially explaining this hyperenhancement and its association with better prognosis.
Radiomics Signatures and Quality Assessment in ICC Prediction Models
Six radiomics signatures were used to formulate a radiomics model, suggesting that differences in tumour heterogeneity between TLSs-positive and TLSs-negative tumours might correlate with distinct radiomics signatures. Prior studies highlighted the potential of radiomics in extracting tumour characteristics and predicting ICC outcomes. Using the Radiomics Quality Score (RQS), this study scored 16, indicating high quality compared to existing studies on cholangiocarcinoma. Notably, the study's innovative focus on predicting TLSs, combined with an external validation cohort, enhances its quality. However, the study has limitations, including its retrospective nature leading to potential selection bias, a limited sample size, and the need for a more extensive cohort for validation. The radiomics feature extraction only considered the portal vein phase, with plans to expand to other phases in subsequent studies.
The study proposes that a CT radiomics nomogram can effectively predict intra-tumoral TLSs status preoperatively, surpassing individual radiomics or clinical models. This nomogram offers improved recurrence-free survival stratification for ICC patients compared to postoperative TLSs status.
Source & Image Credit: Insights into Imaging
Image Credit: iStock