ICU Management & Practice, ICU Volume 14 - Issue 3 - Autumn 2014
The impact of a positive fluid balance on morbidity and mortality has been well established. However, little is known about how to monitor fluid status and fluid overload. This article discusses the different parameters related to bio-electrical impedance analysis (BIA) and their use to monitor fluid status and to guide fluid management in critically ill patients.
Introduction
Bio-electrical impedance analysis (BIA) measures whole body (or regional) impedance, phase angle, resistance, reactance and capacitance, by means of an electric current transmitted at different frequencies (Bioelectrical impedance analysis 1994; Plank et al. 1995). New techniques allow measurement of total body water with separation into extracellular and intracellular water (Janssen et al. 1997). Previous and some recent data suggest that BIA may provide useful information not only in different well-established patient groups (dialysis, AIDS, malnutrition), but also in critically ill patients with burns, trauma and sepsis undergoing fluid resuscitation (Van Biesen et al. 2011; Wabel et al. 2009; Wabel et al. 2008; Plank et al. 2000; Savalle et al. 2011; Kraemer 2006; Kraemer et al. 2006; Streat et al. 1985).
BIA principle
BIA allows calculation of body composition and volumes by means of a current going through the body considered as a cylinder (see Figure 1, page 16). Details of the principles can be found elsewhere (Bioelectrical impedance analysis 1994; Foster and Lukaski 1996; Kyle et al. 2004a; 2004b). In order to obtain reproducible measurements BIA has to make five assumptions: 1) the human body can be considered as a cylinder; 2) this cylinder consists of five smaller cylinders (one central cylinder and two arms, two legs); 3) body composition is assumed to be homogenous; 4) with absence of individual variation, and 5) without impact of environment (temperature, stress, infusions…). This of course only holds true in an ideal situation that may differ from real life situations, especially in the critically ill. To obtain a good BIA measurement five factors are indispensable: impedance value, height, weight, gender and age. Of these gender and age are the most important in obtaining the highest level of accuracy. BIA allows a four compartment body composition analysis dividing the body into fat, water, mineral and protein components (see Figure 2, page 16)(Foster and Lukaski 1996).
BIA Measurement
Reproducible measurements can be obtained with tetrapolar
electrodes with two current electrodes (to drive electricity into the human
body) and two detection electrodes (to detect impedance) placed on hands and
feet. Tetrapolar techniques provide more reproducible results (Kyle et al.
2004; 2004b). Modern devices also apply multiple frequencies, further improving
the reproducibility and accuracy of the results. The frequency is the number of
repetitions per second of a complete electric waveform (1 repetition per second
is 1 Hz)(Plank et al. 1993; Janssen et al. 1997; Streat et al. 2000). A current
with a frequency below 100 Hz will not pass the cell membranes and as such will
measure only extracellular water (ECW). Current frequencies above 100 Hz will
go through cells and measure total body water (TBW). The intracellular water
(ICW) can then be calculated as TBW minus ECW. When electric current passes a
cell membrane a time delay occurs, expressed as phase angle. A phase angle of 0
degrees is an indicator of absence of cell membranes, whereas 90 degrees
represents a capacitive circuit which consists of only membranes with no fluid.
The greater the number of cell membranes the signal has to pass through, the longer the time delay. A high phase angle
hence is consistent with high reactance and a large amount of waste cell membrane
and body cell mass (BCM) as seen in healthy individuals, whereas (critically ill)
patients tend to have a low phase angle (see Figure 3,page 16)( Savalle et al. 2012;
Kyle et al. 2002). Pitfalls one has to take into account during BIA measurement
are changing posture (best position being supine), incorrect position of arms
(should be next to body), incorrect contact with the electrodes and contact
with another person or object during measurement. Other factors that may
interfere with BIA measurements that are currently not yet well understood are:
infusions with large amounts of normal saline, peripheral oedema, changes in
ambient air and skin temperature, sweating, conductance of hospital bed, etc.
BIA Parameters
Table
1 lists the different parameters that can be obtained with BIA. Absolute
measurements of impedance, reactance, resistance and capacitance have been
highly correlated to changes in the human body, and have been shown to be good
prognostic indicators. Under- and overestimation of dry weight is important,
and has been shown to impair the survival and quality of life of haemodialysis patients.
Body composition and nutritional assessment of children and adults in clinical
settings is important in order to identify potential causes of inadequate
nutrition status, including the risk of malnutrition. Performing nutritional
assessments in diseased patients enables medics to identify related disorders
and to monitor the effects of treatment. The glomerular filtration rate (rate
at which waste is removed from our kidneys) is an important indicator of kidney
function. Creatinine estimations can also be performed. BIA can obtain
information on minerals (bone, soft tissue) and protein content of the body. In
some patients assessment of the loss of minerals can be very important. Glycogen
mass is the primary storage form of carbohydrates found in the cytoplasma of
most cells. Intracellular and extracellular body fluid status in both healthy and
diseased patients is of significant importance. Extracellular water (ECW)
increases in different diseases and oedema is the most common sign of ECW
expansion. Body cell mass (BCM) is an accurate method of establishing a healthy
subject’s nutritional status or a patient’s degree of malnutrition.
Validation
Although
BIA is a simple, noninvasive, rapid, portable, reproducible, and convenient method
of measuring body composition and fluid distribution, it is still unclear
whether it is sufficiently accurate for clinical use in critically ill patients
(Aghdassi et al. 2001). BIA measures TBW and as such it needs to be validated
through comparison with other means of determining TBW or methods (such as
densitometry) used to derive the components of the two-compartment model of the
body composition, namely fat free mass and body fat. Because BIA
disproportionately considers the extremities, the relationship between
impedance and TBW can only be empirical (Bioelectrical impedance analysis
1994). This relationship probably exists in most normal subjects and in those
with mild disease perturba tions such as mild-to-moderate obesity and other
chronic illnesses not producing local fluid accumulation.
The gold standard techniques for measuring body composition and TBW are isotope dilution (labelled deuterium), followed by dual energy X-ray absorptiometry (DEXA), underwater weighing, and air-displacement plethysmography. Abdominal and visceral fat can also be measured with CT and MRI. Previous studies showed varying results and in some cases BIA could not provide additional information (Plank et al. 2005; Beshyah et al. 1995; Bracco et al. 1996; Genton et al. 2002). Total body potassium (TBK) has been found to be linearly correlated with body cell mass (BCM) ( Beshyah et al. 1995). Portable methods consist of infrared, skinfold caliper, and dual contact BIA. Tetrapolar BIA falls in between basic and advanced methods. Different studies compared BIA with DEXA showing good correlation. For clinical purposes two devices are available to be used in (critically ill) patients: the Fresenius Medical Care (BCM) Body Composition Monitor (Fresenius Medical Care, Bad Homburg, Germany) (http://www.bcm-fresenius.com/index.html) and the Maltron BioScan 920 (Maltron International Ltd, Rayleigh, Essex, UK) (http://www.maltronint.com/products/bioscan920-2S.php). However, no head-tohead comparison has been performed in a large sample of critically ill patients.
Clinical Applications
As suggested by ESPEN: “BIA is an important clinical tool for evaluating the metabolic status of ICU patients. It is inexpensive and noninvasive, and it provides useful information concerning altered body composition and membrane potential at the tissue level measured by phase angle, as well as fluid imbalance” (Kyle et al. 2004a; 2004b). From a theoretical point of view the clinical applications for BIA could be numerous: AIDS, muscle wasting, anorexia, postmenopausal women, obesity, pregnancy, Crohn’s disease, cystic fibrosis, diabetes, paediatric diseases, enteral and parenteral nutrition, elderly, rheumatoid disease, tropical disease… BIA can be helpful in burn patients, cardiovascular disease, peripheral oedema, gastroenteritis, haemodialysis, CVVH, liver disease, second and third spacing (segmental analysis), lung disease, ARDS, malnutrition, bariatric surgery, postoperative fluid status, renal failure, stroke…
BIA can detect changes in body composition even in the early stages of kidney disease and in patients with cardio-renal syndromes, showing lower resistance, abnormal impedance vectors, reduced phase angle, and higher total body water together with a lower body cell mass. Importantly, patients do need to have overt signs of overhydration or malnutrition for BIA to detect these alterations (Bellizzi et al. 2006; Aspromonte et al. 2012). These changes in body composition continue during the entire spectrum of chronic kidney disease, being most evident in end-stage renal disease (Dumler and Kilates 2003). In chronic haemodialysis patients multifrequency whole body BIA can give an objective measure of fluid and nutritional status, calculating overhydration within one to two litres (Tattersall 2009). Using BIA as a guide to achieving dry weight results in an improved fluid status and control of blood pressure (Moissl et al. 2013). In peritoneal dialysis patients, who often have a significant residual kidney function, BIA guided fluid management (with measurements performed after draining the intraperitoneal cavity (Arroyo et al. 2014) can help restore diuresis in underhydrated patients and improve tension and weight control in overhydrated patients ( Van Biesen et al. 2011; Crepaldi et al. 2009).
Many conditions exist in critical illness (ascites, anasarca, severe peripheral oedema, pleural effusions, the massively overhydrated patient…) where conventional BIA may only provide a poor measure of TBW (Kyle et al.2004a; 2004b). Therefore, for the time being BIA can only be considered as a research tool in critically ill patients because the TBW-to-FFM ratio is variable and the body impedance-to-TBW ratio may often vary during the above-cited conditions ( Bioelectrical impedance analysis 1994; Kyle et al. 2004a; 2004b).
It is important for the clinician to be aware that the normal ECW/ICW ratio is less than 1. Critically ill patients, especially those with severe sepsis, are prone to develop changes in body fluid distribution with migration of fluid from the intravascular to the extravascular space. Furthermore, the systemic inflammatory response produces changes between the FFM and TBW distribution (Harrison 2010). Raw impedance data can provide information on hydration and cell mass integrity (Barbosa-Silva and Barros 2005).
Plank found that although changes in TBW were similar, patients with peritonitis and sepsis (n=12) had higher ECW values compared to those with blunt trauma (n=18) (Plank and Hill 2000). In a study on the use of continuous veno-venous hemofiltration to adjust fluid volume excess in 30 septic shock patients with AKI Dabrowski and co-authors found a sustained increase in nonsurvivors (Dabrowski et al. 2014). A similar study in 68 ICU patients with sepsis (n=51) compared to patients without sepsis (n=17) showed that ECW and FFM hydration were increased in severe sepsis compared to sepsis (Słotwiński et al. 2013). Changes in tissue physiology and integrity during sepsis may produce changes in electrical properties, as such; the use of raw data obtained with BIA seems promising. Indeed, raw data are not influenced by assumptions that can affect body composition results, and bio-electrical impedance vector analysis provides information on tissue hydration and BCM independent of regression equations, even in overhydrated patients (Nwosu et al. 2013). The study by Słotwiński showed that patients with sepsis had significantly higher raw impedance (566}98.66 Ù vs. 423.86}149.7 Ù; p=0.0003) and resistance normalised by height (336.69}66.9 Ù/m vs 259.94}90.9 Ù/m; p=0.00165) than those with severe sepsis (Słotwinski et al. 2013). The lower mean phase angle of the patients with sepsis laying above 50th percentile in the study by Słotwiński could be related to a low body cell mass and high ECW/ICW ratio, as observed by other investigators (Buffa et al. 2013). A retrospective study comparing BIA data from critically ill patients (n=15) with healthy volunteers (n=25) showed significant differences in body water composition between patients and healthy individuals (Huygh et al. 2013). In this study patients had higher values for TBW (45}7.7 vs 38}9.7 Ltr, p=0.01), ECW (24.1}5.4 vs 16.9}5.3 Ltr, p<0.0001) and ECW/ICW ratio (1.2}0.2 vs 0.8}0.2, p<0.0001) while ICW was lower 20.9}3.8 vs 21.2}5 Ltr, p=NS) (Huygh et al. 2013).
Conclusions
BIA is non-invasive and relatively inexpensive. BIA can be performed at bedside, and does not expose to ionising radiation. Modern devices have very limited between-observer variations. However, BIA parameters are population- specific and one must be aware of clinical situations that may interfere with the measurement. BIA allows assessment of TBW, ICW, ECW, ECW/ICW ratio and calculation of volume excess. As such it can help to guide de-resuscitation in patients not transgressing spontaneously from the Ebb to Flow phase of shock (Cordemans et al. 2012). More research is needed in critically ill patients before widespread use of BIA can be suggested in critically ill patients.

Figure 1. BIA Principle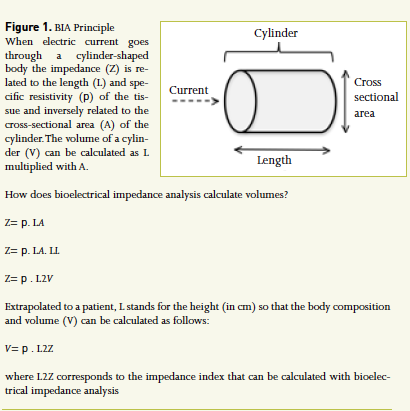
Figure 2. The Four Compartment Body Composition Analysis With BIA
ICW: Intracellular water (body water that exists inside the cell membrane)
ECW: Extracellular water (body water that exists outside the cell membrane Extracellular can be further subdivided into interstitial, lymphatic, trans-cellular fluid and blood)
TBW: Total body water = ICW + ECW (Body water that exists in- and outside of cell membrane)
SLM: Soft lean mass = total body water + protein (Skeletal and smooth muscle maintaining body function)
SMM: Skeletal muscle mass
LBM: Lean body mass = SLM + minerals
BW: Body weight = LBM + body fat
The values in between () are the range of standard body composing constituents. Units are expressed in Litres.
Figure 3. BIA Principles
Panel A. Biological tissues act as conductors or insulators and the flow of current through the body will follow the path of least Resistance (R). Fat Free Mass (FFM) contains large amounts of water and electrolytes and therefore is a better conductor of electrical current than fat and bone, which are poor conductors as they contain low amounts of fluid and conducting electrolytes. The cell membrane of a body consists of a layer of non-conductive lipid (fat, oils and other lipid like substance) material sandwiched between two layers of conductive protein molecules. Cell membranes become reactive elements behaving as capacitors (C) when
electrical current is applied.
Panel B. With BIA the Phase angle (θ), the Reactance (X) and the Resistance (R) are measured. Normal Phase angle is 4-15°. Adapted from Foster and Lukaski.
Acknowledgements
MLNG consults for Maltron and is member of the medical advisory board of Pulsion Medical Systems. This article is a selection of a full review paper published under the Open Access CC BY Licence in Anesthesiology and Intensive Therapy.References:
Arroyo D, Panizo N, Abad S et al. (2014) Intraperitoneal fluid overestimates hydration status assessment by bioimpedance spectroscopy. Perit Dial Int, Mar 1 [Epub ahead of print]
Aspromonte N, Cruz DN, Ronco C et al. (2012) Role of bioimpedance vectorial analysis in cardio-renal syndromes. Semin Nephrol, 32(1): 93-9.
Barbosa-Silva MC, Barros AJ (2005) Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care, 8(3): 311-7.
Bellizzi V, Scalfi L, Terracciano V et al. (2006) Early changes in bioelectrical estimates of body composition in chronic kidney disease. J Am Soc Nephrol, 17(5): 1481-7.
Beshyah SA, Freemantle C, Thomas E et al. (1995) Comparison of measurements of body composition by total body potassium, bioimpedance analysis, and dual-energy X-ray absorptiometry in hypopituitary adults before and during growth hormone treatment. Am J Clin Nutr, 61(6): 1186-94.
Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement, December 12-14, 1994. Bethesda, MD: U.S. Department of Health and Human Services.
Bracco D, Thiébaud D, Chioléro RL et al. (1996) Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J Appl Physiol, 81(6): 2580-7.
Buffa R, Saragat B, Cabras S et al. (2013) Accuracy of specific BIVA for the assessment of body composition in the United States population. PLoS One, 8(3): e58533.
Cordemans C, De Laet I, Van Regenmortel N et al. (2012) Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care, 2 Suppl 1: S15.
Crepaldi C, Soni S, Chionh CY et al. (2009) Application of body composition monitoring to peritoneal dialysis patients. Contrib Nephrol, 163: 1-6.
Dabrowski W, Kotlinska-Hasiec E, Schneditz D et al. (2014) Continuous veno-venous hemofiltration to adjust fluid volume excess in septic shock patients reduces intra-abdominal pressure. Clin Nephrol 82(7): 41-50.
Dumler F, Kilates C (2003) Body composition analysis by bioelectrical impedance in chronic maintenance dialysis patients: comparisons to the National Health and Nutrition Examination Survey III. J Ren Nutr, 13(2):166-72.
Foster KR, Lukaski HC (1996) Whole-body impedance--what does it measure? Am J Clin Nutr, 64(3 Suppl): 388S-96S.
Genton L, Hans D, Kyle UG, Pichard C (2002) Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition, 18(1): 66-70.
Harrison C (2010) Sepsis: calming the cytokine storm. Nat Rev Drug Discov, 9(5): 360-1.
Huygh J, De Vos J, Hofkens P-J et al. (2013) Comparison of bio-electrical impedance analysis in healthy volunteers and critically ill patients. Fluids, 2(2): 185-6.
Janssen YJ, Deurenberg P, Roelfsema F (1997) Using dilution techniques and multifrequency bioelectrical impedance to assess both total body water and extracellular water at baseline and during recombinant human growth hormone (GH) treatment in GH-deficient adults. J Clin Endocrinol Metab, 82(10): 3349-55.
Kraemer M (2006) A new model for the determination of fluid status and body composition from bioimpedance measurements. Physiol Meas, 27(9): 901-19.
Kraemer M, Rode C, Wizemann V (2006) Detection limit of methods to assess fluid status changes in dialysis patients. Kidney Int, 69(9): 1609-20.
Kyle UG, Bosaeus I, De Lorenzo AD et al. (2004a) Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr, 23(6): 1430-53.
Kyle UG, Bosaeus I, De Lorenzo AD et al. (2004b) Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr, 23(5): 1226-43.
Kyle UG, Unger P, Dupertuis YM et al. (2002) Body composition in 995 acutely ill or chronically ill patients at hospital admission: a controlled population study. J Am Diet Assoc, 102(7): 944-55.
Moissl U, Arias-Guillén M, Wabel P et al. (2013) Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol, 8(9): 1575-82.
Nwosu AC, Mayland CR, Mason SR et al. (2013) Hydration in advanced cancer: can bioelectrical impedance analysis improve the evidence base? A systematic review of the literature. J Pain Symptom Manage, 46(3): 433-46.e6.
Plank LD, Hill GL (2000) Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann N Y Acad Sci, 904: 592-602.
Plank LD, Monk DN, Gupta R et al. (1995) Body composition studies in intensive care patients: comparison of methods of measuring total body water. Asia Pac J Clin Nutr, 4(1): 125-8.
Savalle M, Gillaizeau F, Maruani G et al. (2012) Assessment of body cell mass at bedside in critically ill patients. Am J Physiol Endocrinol Metab, 303(3): E389-96.
Słotwiński R, Milewska M, Kosałka K et al. (2013) Raw impedance data analysis in severe ill patients with sepsis. Fluids, 2(2): 168-70.
Streat SJ, Beddoe AH, Hill GL (1985) Measurement of total body water in intensive care patients with fluid overload. Metabolism: clinical and experimental, 34(7): 688-94.
Streat SJ, Plank LD, Hill GL (2000) Overview of modern management of patients with critical injury and severe sepsis. World J Surg, 24(6): 655-63.
Tattersall J (2009) Bioimpedance analysis in dialysis: state of the art and what we can expect. Blood Purif 27(1): 70-4.
Van Biesen W, Williams JD, Covic AC et al. (2011) Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One, 6(2): e17148.
Wabel P, Chamney P, Moissl U et al. (2009) Importance of whole-body bioimpedance spectroscopy for the management of fluid balance. Blood Purif, 27(1): 75-80.
Wabel P, Moissl U, Chamney P et al. (2008) Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant, 23(9): 2965-71.





















