ICU Management & Practice, Volume 17 - Issue 1, 2017
EEG measures continuously at the bedside the human brain’s electrical activity. Its main advantages are noninvasiveness, good spatial and temporal resolution, and sensitivity to changes in both brain structure and function.
In ICU, seizures are frequent in patients with/without acute brain injury. They are often difficult to recognise, because they are non-convulsive. This provides support in favour of continuous EEG (cEEG) rather than ‘‘spot’’ EEG, typically for a period of less than 30 minutes. cEEG refers to the recording of EEG over extended time periods in critically ill patients at risk for secondary brain injury and neurologic deterioration. Unfortunately, intensivists aren’t usually trained in interpreting EEG, due to difficulties in interpretation of the recordings. Usually a neurophysiologist’s consult is required. For this reason, EEG has been usually recorded on the spot, and it has not been deemed a potentially useful tool for continuous monitoring of the damaged brain, except in some neurological institutions. Fortunately, times are changing.
Indications for Continuous EEG Recording in ICU
Several guidelines recommend the use of cEEG
in the ICU setting (Claassen et al. 2013; Le Roux et al. 2014; Claassen and
Vespa 2014; Herman et al. 2015) for:
1.The
diagnosis of nonconvulsive seizures(NCS)
and nonconvulsive status epilepticus(NCSE).
NCSE is a state of continuous/repetitive seizures without convulsions. Due
to the nonspecific signs and significant morbidity and mortality associated
with NCSE, research has focused on early diagnosis and seizure termination.
Standard EEG misses identifying NCSE in more than 75% of cases. Instead cEEG
(lasting 6-12 hours or longer) is able to identify up to 80% of NCSE (Friedman et
al. 2009). NCS are associated with such secondary insults as increased
intracranial pressure, reduction in tissue oxygenation, and local metabolic
derangements. Therefore, even if the effect of NCS identification and management
on outcome has not been fully proven, untreated NCS is associated with increased
mortality and increased risk for poor neurologic outcome. Therefore early
identification of NCS with cEEG in patients with unexplained altered level of
consciousness is strongly recommended (Sutter et al. 2016).
2.The
assessment of efficacy of therapy forseizures
and status epilepticus, when sedation and high-dose suppressive therapy,
after firstline therapies, are required.
3.
The identification of cerebral ischaemia. cEEG could detect delayed
cerebral infarction in subarachnoid haemorrhage patients before clinical
deterioration and CT scan changes become evident. In fact, the reduction of the
ratio between alpha (8-13 Hz) and delta (<4 Hz) frequency, with an increase
in slow frequencies, is becoming an interesting application of cEEG (Claassen
et al. 2004; Rots et al. 2016).
4.The expansion of multimodality monitoring(MMM) of the injured brain, adding continuous information on the function of the cerebral cortex and its metabolic and functional variations. Advanced MMM should integrate neurophysiological information with neuroimaging and different continuous physiologic data, such as ICP, CPP, and PbtO2, with EEG-derived parameters (Citerio et al. 2015).
Barriers and Possible Solutions for Implementing cEEG in ICU
Even if the indications are rather clear,
ICU practice is far from a diffuse implementation of cEEG. Barriers to its
implementation are here summarised, along with possible solutions for
overcoming these obstacles:
- Limited availability of EEG technicians andneurophysiologists to review the studies24/7.
In the UK, for example, a survey documented that only a minority of ICU units (33%) have access to continuous EEG monitoring, despite it being considered fundamental for patients’ management (Patel et al. 2015). In a larger USA survey, continuous EEG is more frequently utilised (Gavvala et al. 2014). However, a substantial interhospital variability has been described. In a single centre study, only a minority (27%) of critically ill patients presenting criteria for EEG monitoring had an EEG recording (Park and Boyd 2015).
A possible solution is the integration of ICU staff in the continuous evaluation of the EEG recording. In our unit, after a standard EEG, EEG technicians position the electrodes with ICU nurses’ help. Nurses have been trained to check the recording hourly and to reposition the electrodes if not working and to use a transparent dressing for stabilising the electrodes over time. Daily check of the system is planned by neurophysiologist technicians. Neurophysiologists discuss with ICU doctors the indications for monitoring and, on a daily basis, discuss the 24-hour recordings with ICU staff. ICU doctors and residents, present 24/7, have been trained to identify the most significant patterns utilising derived parameters as quantitative EEG (see below).
- Lack of uniform terminology and ofconsensus on the clinical significance ofselected EEG patterns.
Neurophysiologists defined some criteria (Leitinger et al. 2015) for NCS identification, but variability in EEG interpretation still remains (Rodriguez Ruiz et al. 2016). Intensivists using cEEG need to focus on important items, i.e. outcome-related patterns. In our experience, we targeted to identify during the cEEG monitoring phase:
o Artefacts
o Seizures
o Sedation level
o Asymmetry
- Need for an infrastructure for cEEG in abusy modern ICU environment.
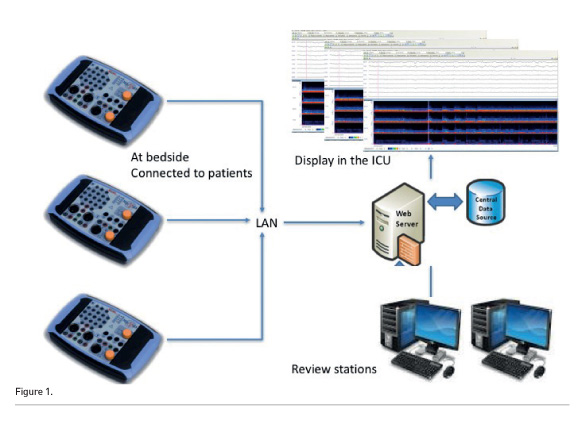
Ideal tools are cheap, small, capable of being fully networked, with the possibility to review the recording in several locations. Figure 1 presents the setting actually used in the San Gerardo Neurointensive Care Unit. Several small EEG patients’ units are networked. In the ICU, the recordings are displayed at nursing and medical stations. Review stations, two in the ICU offices and the third in the neurophysiologists’ offices, are available. All the data are stored on a dedicated server.
- Huge
amount of recorded EEG-data requires time for reviewing and interpretation.
If we want to utilise cEEG as a monitoring tool,
continuous evaluation of these recordings is needed. Moreover, for making the
monitoring useful in the patient’s care plan, intensivists, while detecting a
pathological condition (i.e. seizures or oversedation), have to react, modifying
their therapeutic approach.
See Also:Multimodality Neuromonitoring in Critically Ill Patients
Implementation
of qEEG: Summary of Our Experience
We studied the implementation of qEEG in our Unit in the clinical trial Continuous Quantified EEG in NeuroIntensive Care (CrazyEEG), NCT02901262 (clinicaltrials.gov/ct2/show/ NCT02901262), following these steps:
Phase 1. Definition of a cEEG recording setting.
The neurophysiologists and the intensivists defined a common setting for the study. Continuous EEG was recorded using 8 electrodes arranged according to the 10-20 International System, on a bipolar longitudinal montage plus a ground and a reference electrode.
Our c/qEEG setting includes:
- Continuous raw EEG tracings, useful for the neurophysiologist check of the Density Spectral Array (DSA) data,
- DSA is an EEG power-based display used to convey the frequency and power distribution of the EEG signal over time.
- Amplitude-integrated EEG (aEEG). Amplitude- integrated EEG expresses the amplitude compressed on a logarithmic scale of the EEG with an upper margin and a lower margin showing the highest and the lowest amplitude of EEG in a time period. It represents the collective electrical energy of neuronal firing.
- Burst suppression rate (BSR), measuring the amount of time within an interval spent in the suppressed state. This ratio increases as the brain becomes progressively less active, and it is an indicator of pharmacological suppression intensity.
Figure 2 depicts a raw EEG (30 sec) and in the same patient. In the bottom part of the figure, the Density Spectral Array (DSA) is obtained from raw data using a Fourier transformation that gives the power contained within the various frequency bands, and is represented on a x-y graph that shows the time on the x axis and colours corresponding to the power at different frequencies on the y axis.
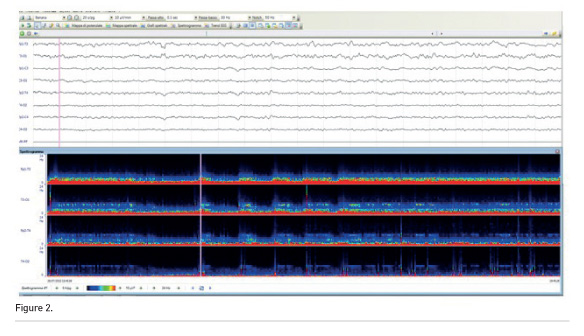
cEEG was recorded accordingly to the previously presented guidelines, including unexplained neurological status based on clinical history and imaging, frequent seizures and status epilepticus suspicion and management.
Phase 2. Baseline evaluation and neurophysiology training.
Intensivists were exposed to online training using the Clinical Electroencephalography for Anesthesiologists presentation developed by Purdon and Brown at Massachusetts General Hospital (https://iii.hm/7x4).
We anonymously tested the baseline knowledge on qEEG after the online course using a web-based system. Ten recordings with the display defined in step 1 were randomly presented.
We evaluated the ability to:
- assess the depth of sedation
- evaluate symmetry between the hemispheres
- recognise seizures and
- recognise artefacts
The responses from intensivists were compared to those of two experienced neurophysiologists, used as “gold standard”. We were disheartened after this step. Intensivists were not so good in interpreting qEEG.
After the baseline test, the intensivists received formal neurophysiology training consisting of lectures and discussion with the neurophysiologist of the recorded qEEG, integrating qEEG data with the clinical status and management strategies of the patients.
Phase
3. Check of the interpretation of qEEG after a 6-month learning period.
We compared qEEG evaluation by intensivists with the neurophysiologists’ interpretation after 6 months of exposition and daily discussion. An app was developed for this aim (Figure 3). Every 12-24 recording period has been evaluated by the intensivist and by the neurophysiogist independently and blindly. We compared the responses after the first 25 patients.
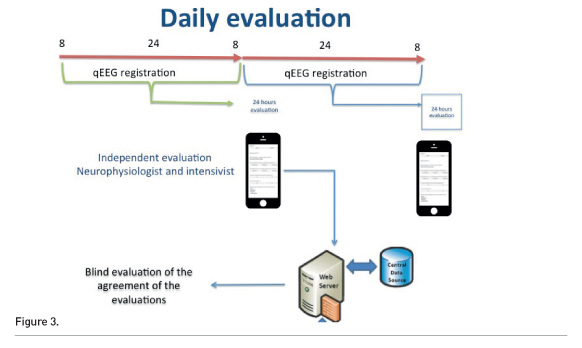
The depth of sedation was correctly evaluated by intensivists in 90.7% of cases, artefacts in 95.3% of cases, symmetry in 81.4% of cases and seizures in 80.2% of cases.
Conclusions
The implementation of a qEEG system, supported by frequent interaction with a neurophysiologist, boosted the use of cEEG in our ICU.
ICU physicians cannot fully substitute for a neurophysiologist. Nevertheless, if they focus on clinically relevant questions (i.e. presence of seizures) they can gain sufficient knowledge to identify potentially dangerous conditions and for starting timely treatment.
Conflict of Interest
Giuseppe Citerio declares that he has no conflict of interest.
Abbreviations
aEEG amplitude-integrated EEG
cEEG continuous EEG
BSR Burst suppression rate
c/qEEG continuous quantitative EEG
CPP cerebral perfusion pressure
DSA Density Spectral Array
EEG electroencephalogram
ICP intracranial pressure
MMM multimodality monitoring
NCS nonconvulsive seizures
NCSE nonconvulsive status epilepticus
PbtO2 partial pressure of brain tissue oxygen
qEEG quantitative EEG




















