ICU Management & Practice, Volume 20 - Issue 4, 2020
Introduction
It is plausible that the importance of nutrition may differ across different phases of illness. Reflecting this, the most recently updated clinical nutrition practice guidelines for critical care from the European Society for Clinical Nutrition and Metabolism (ESPEN) recommend tailoring nutrition provision according to illness phase. The phases are defined as: 1) acute phase, early period (days 1-2); 2) acute phase, late period (days 3-7) and; 3) late/chronic phase (after day 7) (Singer et al. 2019). There are limited recommendations for the nutritional management of patients in the chronic phase of critical illness, and beyond Intensive Care Unit (ICU) admission.
The acute phase of critical illness is characterised by an altered metabolic response, including mobilisation of endogenous glucose stores, hyperglycaemia, hypertriglyceridaemia and protein catabolism, leading to changes in metabolic rate, body composition, reduced muscle mass and function (Merriweather 2020; Massanet et al. 2015; Preiser et al. 2015). Nutrition focussed research in this period has failed to show benefit (and one large study indicated harm), possibly due to the length of intervention, lasting approximately 7 days (Target Investigators 2018; Casaer et al. 2011).
In the chronic phase of critical illness, occurring after day 7 in ICU or out on the ward following ICU discharge, critically ill patients may be more physiologically able to process nutrients, possibly making this a key time for nutrition to support recovery (Wischmeyer 2017). During this phase, the body experiences a substantial increase in metabolic requirements and total energy expenditure (Wischmeyer 2017). Therefore, failure to provide adequate nutrition in this phase could negatively affect skeletal muscle mass, physical ability, or functional recovery (Bear et al. 2017). Furthermore, given that the acute phase only represents a small proportion of the patient journey, it seems logical that the next step for nutrition research should be to investigate the effect of a nutrition intervention provided in both the acute and chronic phases following critical illness.
Evidence from outside of critical care show promise for an intervention that covers the whole hospitalisation period. Conducted in eight ICUs in Switzerland, a recent randomised controlled trial (RCT) including 2088 elderly patients, investigated specialised nutritional support versus standard hospital food provision across whole acute hospitalisation (Scheutz et al. 2019). Compared to standard hospital food provision, those who received individualised nutritional support after 30 days, had lower rates of adverse clinical outcomes and better survival rates.
What Do We Know So Far?
It is well documented that adequacy of nutrition in the acute phase of ICU is below clinician estimates. A retrospective analysis of the International Nutrition Survey data from 2007-2013 including 17,154 patients worldwide who received enteral nutrition (EN) and/or parenteral nutrition (PN), showed that only 56% energy targets were achieved and 52% protein (Ridley et al. 2018). This is consistent with other studies and practice has largely remained unchanged in the past decade (Cahill et al. 2010; Bendavid et al. 2017; Rougier et al. 2020).
And for those who exclusively eat orally within the ICU, less energy and protein are received than those who receive artificial or combination nutrition therapies. In a single-centre observational study conducted in Germany, intake was measured in 289 patients within a mixed medical and surgical ICU (Rougier et al. 2020). In the 126 patients who received oral nutrition only, they received poorer energy and protein intake than the overall study average. Furthermore, of the oral-only patients who had an ICU stay of ≥7 days (n=37), 51% never received ≥80% of their energy targets and 94% never received ≥80% of their protein targets (Rougier et al. 2020).
There are also documented issues immediately post extubation. One of the first reports, a single centre observational study from the Unites States, reported in 50 patients that average energy and protein intake failed to exceed 55% of predicted requirements on any day in the 7 days post extubation (Peterson et al. 2010).
Multiple barriers to oral intake were reported, including mental status (47%), appetite (38%), nausea/vomiting (26%), and therapeutic (restrictive) diets (22%). In New Zealand, an observational study of 79 patients followed critically ill patients upon commencement of oral intake and identified inadequate oral intake in 62% (n=49). For most patients, this occurred early in ICU admission with 25% continuing to experience poor oral intake beyond ICU day 5 (Jarden et al. 2020). More recently, an observational study of 19 patients quantified energy and protein intakes for up to 14 days following liberation from mechanical ventilation (Moisey et al. 2020). The median [IQR] amount of protein and energy received compared dietitian prescription was 46% [74] and 71% [62], respectively. However, on days oral diet was the sole source of nutrition, median intakes compared with prescription were 27% [26] for protein and 47% [37] for energy.
Similar issues have been documented following ICU discharge. In an Australian single-centre prospective observational study of 37 patients with a traumatic brain injury, energy and protein deficits were larger on the ward than during ICU admission and adequacy was lower in those who received oral nutrition compared to EN (75 (37)% energy and 74 (40)% protein vs 89 (34)% energy and 76 (34)%, respectively) (Chapple et al. 2016).
Similarly, a second Australian cohort study examined nutrition intake in 32 patients (predominantly cardiac and trauma diagnosis) from 2 centres on the ward following ICU discharge and found the lowest median [IQR] adequacy was achieved on days where oral nutrition was received with no supplementation (37 [21-6]% energy and 48 [13-63]% protein) and the highest when oral nutrition was combined with EN (104 [66-132]% energy and 99 [60-127]% protein) (Ridley et al. 2018).
Despite the chronic phase of critical illness being of potential importance in recovery, what is known during this period is concerning; nutrition intake is often worse than in the acute phase. This undernutrition could potentially lead to poor recovery in the long-term and warrants further investigation (Bear et al. 2017; Merriweather 2020).
What is Causing Poor Nutritional Adequacy in the Post ICU Period?
More research is required to fully understand why these issues exist, but they can loosely be divided into patient, clinician and system barriers (Ridley et al. 2020) (Figure 1).
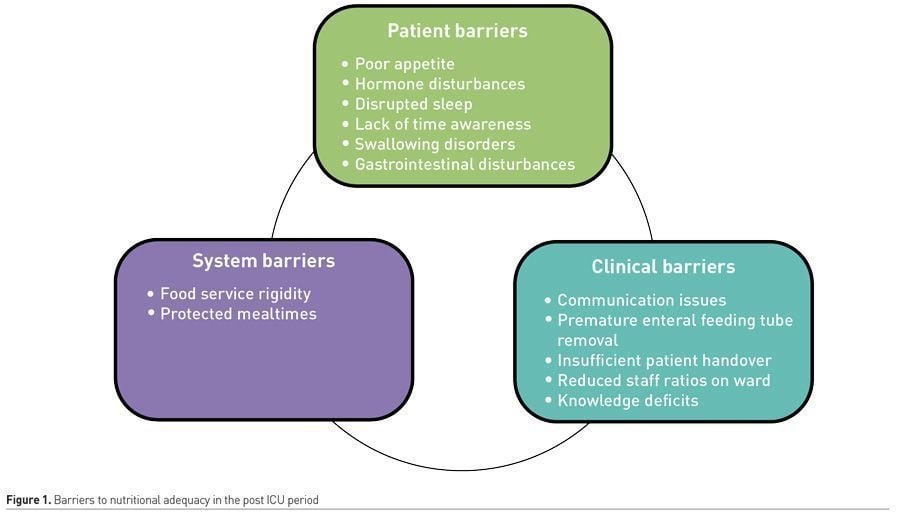
Patient Barriers
Poor appetite
Poor appetite, early satiety, and taste changes are commonly reported as affecting oral intake following extubation, and this can persist throughout acute hospitalisation (Moisey et al. 2020; Peterson et al. 2010; Merriweather et al. 2018). However, the exact mechanism is unclear and likely multifactorial. One study in 16 ICU patients compared to 36 healthy volunteers found lower plasma concentrations of ghrelin (appetite-stimulating hormone) and higher levels of pancreatic peptide YY (PYY) (which acts to reduce appetite) in ICU patients compared to healthy controls. By the fourth week following critical illness, ghrelin had increased, and a positive relation was found with hunger. Alterations in gut hormones could explain changes in oral intake and appetite in the critically ill and should be further explored.
Poor appetite has been associated with longer length of hospitalisation and inflammation. In a cohort of 193 adult ICU survivors, longer length of acute ward admission following ICU and higher levels of serum C-reactive protein were associated with poorer appetite measured using a visual analogue scale (Merriweather et al. 2018).
Irrespective of any hormone changes, it is plausible that the ICU environment itself could affect appetite. For example, disrupted sleep and a lack of awareness of time and the concept of day versus night is likely to disrupt appetite-stimulating hormones leptin and ghrelin (Morselli et al. 2012)
Swallowing disorders
The length of intubation has been associated with dysphagia. A systematic review included 14 studies and indicated that dysphagia, or swallowing disorders, were present in 3-62% of patients following extubation (and in whom were intubated for 125-347 hours prior) (Skoretz et al. 2010). Furthermore, in Europe, in an observational study conducted in two ICUs, 933 patients were analysed and 12.4% (n=116) had dysphagia at 3 hours post extubation, with 10.3% (n=96/933) having dysphagia at ICU discharge (Schefold et al. 2017). Dysphagia at hospital discharge continued in 60% of these patients (n=58/96). In this same study, patients who experienced dysphagia required more days on enteral feeding, had a longer length of mechanical ventilation and hospital stay, and had higher rates of hospital mortality.
Gastrointestinal (GI) disturbances
GI disturbances such as nausea, vomiting, diarrhoea, and high gastric residual volumes are common during the acute phase of critical illness, which if they persist, may lead to a compromised nutritional state (Peterson et al. 2010; Reintam et al. 2009; Chapman et al. 2011; Chapman et al. 2013). An Australian single-centre study compared gastric emptying in 51 ICU survivors three months following ICU discharge with 25 healthy individuals and found no differences in gastric emptying (Chapple et al. 2019). This study suggests that gastric emptying issues that occur during acute phase of critical illness may resolve in the chronic phase.
Clinician Barriers
Some factors affecting nutrition following ICU discharge exist between clinicians or clinical disciplines.
Communication issues
Premature removal of enteral feeding tubes, prior to establishment of adequate oral intake may be an explanation for the poor oral nutrition adequacy in the recovery phase of illness. A single-centre observational study in Scotland conducted in 17 patients reported that 9 patients were transferred with a nasogastric tube (NGT) in situ; 6 were removed within 48 hours of arrival to the ward based on medical staff advice, prior to any formal assessment of nutritional intake by the dietitian (Merriweather et al. 2013).
The National Institute for Health and Excellence (NICE) guideline in Rehabilitation after critical illness recommends that upon discharge from ICU, there should be a handover including the ongoing nutrition treatment plan (NICE 2009). A qualitative study found that this was not routinely provided by nursing, with limited nutrition documentation provided upon transfer from ICU to the ward, and verbal handover only detailing the route of nutrition or commencement of oral intake (Merriweather et al. 2013).
Resource issues
Once a patient is transferred to the ward following ICU discharge, there is often a reduction in nurse to patient ratio. This leads to competing priorities and multiple work-related pressures for nurses and challenges to prioritising nutrition-related care on the ward (Marshall, et al. 2019). This may negatively impact nutrition intake as critically ill patients in the chronic phase of illness are often deconditioned and require feeding assistance and support.
Although oral nutrition is the most common mode of nutrition following ICU discharge, it has been reported in a single-centre study of 37 patients that 71.5% of dietitians time was spent managing patient’s receiving EN and 20.4% of time was oral nutritional management (Chapple et al. 2016). Understanding effective models of nutrition care is important to overcoming some of these issues.
Knowledge deficits
A systematic review including 24 studies synthesised the nutrition education provided to medical students and found that regardless of country, setting or year of medical training, nutrition is insufficiently incorporated into medical education leading to reduced knowledge, skills and confidence in implementing nutrition care to patients (Crowley et al. 2019).
System barriers
Food service times and structure
Qualitative studies have shown that patients feel frustrated with the rigidity of meal service times and the structure of three large meals per day with minimal snack options as it tended to differ from many patients’ usual eating patterns (Merriweather et al. 2013). This rigid structure may impact nutrition intake and ability to meet nutrition requirements orally, however implementing strategies to counteract these issues could improve intake.
A team in Australia implemented a room service model for food selection and delivery in a public hospital where meals are prepared and delivered within 45 minutes of patient orders (McCray et al. 2018). Energy and protein intakes were significantly higher in the room service model than the traditional model and plate wastage was reduced. Patient satisfaction and food costs overall improved significantly.
Protected mealtimes
Many health services have implemented strategies to ensure uninterrupted mealtimes with minimal distractions, however these are not always adhered to (Merriweather et al. 2013). Additionally, mealtimes are protected from family member visits, which could be a further concern for the patients who require feeding assistance due to poor dexterity or poor functional capacity on the ward following ICU discharge.
What Can Clinicians Do To Help Patients Nutritionally in the Post ICU Period?
The ESPEN ICU nutrition guidelines recommend considering any patient that had an ICU admission of 48 hours or more as ‘at risk’ nutritionally (Singer et al. 2019). This recommendation should continue throughout the entire hospitalisation, particularly if known weight loss or muscle wasting has occurred during the acute phase of illness. During the chronic phase of critical illness, it should be considered that additional nutrition supplementation will be required where oral intake is received.
Another change to service provision lies within the dietetic service. Shortfalls exist in nutrition provision following ICU discharge and we are only just beginning to understand that multiple patient barriers exist following extubation or prolonged critical illness. We also know that in the following ICU discharge, there is an increase in physical therapies and the body shifts to a more anabolic stage of recovery (Massanet et al. 2015). Further, it could be hypothesised that meeting nutrition requirements in this phase will ensure rehabilitation occurs; this requires prospective investigation. Given that oral patients get the least amount of time spent on their care yet have the poorest nutrition adequacy, the most effective models of care need to be explored (Chapple et al. 2016; Ridley et al. 2018).
Lastly, nutrition related research in the area of critical illness needs to encompass the chronic phase. There is a discord between current nutrition research that measures long term functional recovery yet delivers short-term nutrition interventions. Furthermore, it is currently unclear what impact specific nutrition interventions may have on recovery in relation to critical illness (Lambell et al. 2020). We are unaware what happens to patients once they are transferred to the subacute setting and once discharged home. The issues and barriers patients face from critical illness are likely to exist for long periods beyond the acute care setting. Therefore, the next logical step is to extend nutrition interventions into the chronic phase following ICU discharge (that is, the post ICU period) to better understand how nutrition effects recovery after critical illness and whether it improves physical function and/or quality of life (Bear et al. 2017).
Conclusion
Nutrition interventions in the chronic phase of critical illness have been identified as a research priority, with this period currently under-investigated. The studies published to date have identified several issues, including gross nutrition inadequacy (particularly with oral nutrition) and some of the factors that affect this. More research is needed in this area to truly understand the impact of nutrition interventions on long-term recovery from critical illness, with a specific focus on the application of longer-term nutrition interventions. In the meantime, there are patient, clinician and system level strategies that could be adopted to improve nutrition intake in patients who are recovering from critical illness across the spectrum of recovery.
Conflict of Interest
None. 
Abbreviations
ICU: Intensive Care Unit
EN: Enteral Nutrition
PN: Parenteral Nutrition
PYY: Pancreatic peptide YY
NGT: Nasogastric Tube
ESPEN: European Society for Clinical Nutrition and Metabolism
Key Points
ESPEN guidelines recommend tailoring nutrition provision according to illness phase.
Nutrition intake is often worse in the chronic phase of critical illness than in the acute phase.
- There are multiple barriers that can affect nutritional adequacy in the post ICU period. These include patient barriers, clinician barriers and system barriers.
- Nutrition interventions in the chronic phase of critical illness are a research priority.
References:
Bear DE, Wandrag L, Merriweather JL, et al. (2017) The role of nutritional support in the physical and functional recovery of critically ill patients: a narrative review, Crit Care, 21: 226.
Bendavid I, Singer P, Theilla M et al. (2017) NutritionDay ICU: A 7 year worldwide prevalence study of nutrition practice in intensive care, Clin Nutr, 36 (4): 1122-29.
Cahill NE, Dhaliwal R, Day AG et al. (2010) Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicentre observational study, Crit Care Med, 38 (2): 395-401.
Casaer MP, Mesotten D, Germans G et al. (2011) Early versus Late Parenteral Nutrition in Critically Ill Adults, N Engl J Med, 365 (6): 506-17.
Chapman MJ, Besanko LK, Burgstad CM, Fraser RJ, Bellon M, O'Connor S et al. (2011) Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement, Gut, 60: 1336-43.
Chapman MJ, Nguyen NQ, Deane AM (2013) Gastrointestinal dysmotility: evidence and clinical management, Curr Opin Clin Nutr Metab Care, 16: 209-16.
Chapple LS, Deane AM, Heyland DK et al. (2016) Energy and protein deficits throughout hospitalization in patients admitted with a traumatic brain injury, Clin Nutr, 35 (6): 1315-22.
Chapple LS, Weinel LM, Abdelhamid YA et al. (2019) Observed appetite and nutrient intake three months after ICU discharge, Clin Nutr, 38: 1215-20.
Brooks R et al. (2003) The Measurement and valuation of health status using EQ-5D: a European prospective. London: Kluwer Academic Publishers Dordrecht.
.
Brück E, Schandl A , Bottai M, Sackey P (2018) The impact of sepsis, delirium and psychological distress on self related cognitive function in ICU survivors-a prospective cohort study. J Intensive Care,.8;6:2. doi: 10.1186/s40560-017-0272-6.
Casaer MP, Wilmer A, Hermans G et al. (2013) Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am. J. Respir. Crit. Care Med, 187: 247–55.
Colbenson GA, Johnson A, Wilson ME (2019) Breathe (Sheff).Post-intensive care syndrome: impact, prevention, and management.15(2):98-101. doi: 10.1183/20734735.0013-2019
Connolly B, Salisbury L, O’Neill B et al. (2015) Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst. Rev, CD008632.
Cullen DJ, Ferrara L, Briggs B, et al. (1976) Survival, hospitalization charges and follow-up results in critically ill patients. NEJM. 294:982–7.
Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ (2008) Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen. Hosp. Psychiatry, 30: 421–34.
Davydow DS, Zatzick D, Hough CL, Katon WJ (2013) In-hospital acute stress symptoms are associated with impairment in cognition 1 year after intensive care unit admission. Ann. Am. Thorac. Soc, 10: 450–7
Demling RH (2009) Nutrition, anabolism, and the wound healing process: an overview. Eplasty, 9: e9.
Devlin JW et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med,.46(9):e825-e873. doi: 10.1097/CCM.0000000000003299.
EQ-5D 3L Users Guide (2018) Version 6.0.
Garrouste-Orgeas M, Coquet I, Perier A et al. (2012) Impact of an intensive care unit diary on psychological distress in patients and relatives*. Crit. Care Med, 40: 2033–40.
Griffiths J, Hatch RA, Bishop J, et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care 2013; 17: R100
Guerra C, Hua M, Wunsch H. Risk of a diagnosis of dementia for elderly medicare beneficiaries after intensive care. Anesthesiology 2015
Harvey MA, Davidson JE. Post-intensive care syndrome: right care, right now…and later. Crit Care Med 2016; 44: 381–385
Hopkins RO, Suchyta MR, Snow GL, Jephson A, Weaver LK, Orme JF. Blood glucose dysregulation and cognitive outcome in ARDS survivors. Brain Inj. 2010; 24: 1478–84
Hosey, M.M., Needham, D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers 6, 60 (2020). https://doi.org/10.1038/s41572-020-0201-1
Inoue S, Hatakeyama J, Kondo Y, et al. Post‐intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019 Jul; 6(3): 233–246
Katz IR, Curyto KJ, TenHave T, Mossey J, Sands L, Kallan MJ. Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am. J. Geriatr. Psychiatry 2001; 9: 148–59.
Kim IY, Schutzler S, Schrader A et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. Endocrinol. Metab. 2016; 310: E73–80.
Lambell KJ, King SJ, Forsyth AK, Tierney AC. Association of energy and protein delivery on skeletal muscle mass changes in critically ill adults: a systematic review. JPEN J. Parenter. Enteral Nutr. 2018; 42: 1112–22
Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011; 10: 931–41.
Mandebvu F, Kalman M. The 3 Ds, and newly acquired cognitive impairment: issues for the ICU nurse. Crit. Care Nurs. Q. 2015; 38: 317–26
McClave SA, Taylor BE, Martindale RG et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016; 40: 159–211
Morton RW, Murphy KT, McKellar SR et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018; 52: 376–84.
Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369: 1306–1316
Phillips SM. A brief review of critical processes in exerciseinduced muscular hypertrophy. Sports Med. 2014; 44(Suppl 1): S71–7.
Pisani MA, Redlich CA, McNicoll L, Ely EW, Friedkin RJ, Inouye SK. Short-term outcomes in older intensive care unit patients with dementia. Crit. Care Med. 2005; 33: 1371–6.
Rawal G, Yadav S and Kumar R. Post-intensive Care Syndrome: an Overview. J TranslInt Med. 2017 Jun; 5(2): 90–92.
Schofield-Robinson O J, Lewis S R, Smith A F, et al. Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Library 2018
Singer P, Blaser AR, Berger MM et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2018; 38: 48–79
Ullman A J, Aitken L M, et al. Cochrane Database Syst Rev. 2014 Dec; 2014(12): CD010468. Diaries for recovery from critical illness.
Van Der SchaafMBakhshi-Raiez F, , Van Der Steen M, Dongelmans DA, De Keizer NF. Recommendations for intensive care follow-up clinics; report from a survey and conference of Dutch intensive cares.. Minerva Anestesiol. 2015 Feb;81(2):135-44.
Warnakulasuriya SR, Patel RC, Singleton GF, Moonesinghe SR. Patient-reported outcomes for ambulatory surgery.CurrOpinAnaesthesiol. 2020 Sep 29. doi: 10.1097/ACO.0000000000000921. Online ahead of print.PMID: 33002956
Wolters AE, Slooter AJ, van der Kooi AW, van Dijk D. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med. 2013; 39: 376–86






















