ICU Management & Practice, Volume 22 - Issue 4, 2022
Antimicrobial resistance (AMR) has been defined as a major threat to healthcare and to humanity by the World Health Organization (WHO 2015). The level of evidence for the association between AMR and hospital deaths, hospital length of stay and healthcare-associated costs is growing. Cassini et al. (2019) reported an increasing and substantial estimated burden of AMR-related infections compared with other infectious diseases, in children, in the elderly, and in the countries of Southern Europe (Italy and Greece) for 16 pathogen-antibiotic combinations. A systematic analysis showed that the global burden associated with AMR infections in 88 pathogen-antibiotic combinations was estimated to be 4.95 million (95% uncertainty intervals (UI) 3.62–6.57) deaths including 1.27 million (95% UI 0.91-1.71) deaths that were directly attributable to AMR (Murray et al. 2022). Of note, the highest rates of death were located in sub-Saharan Africa and South Asia.
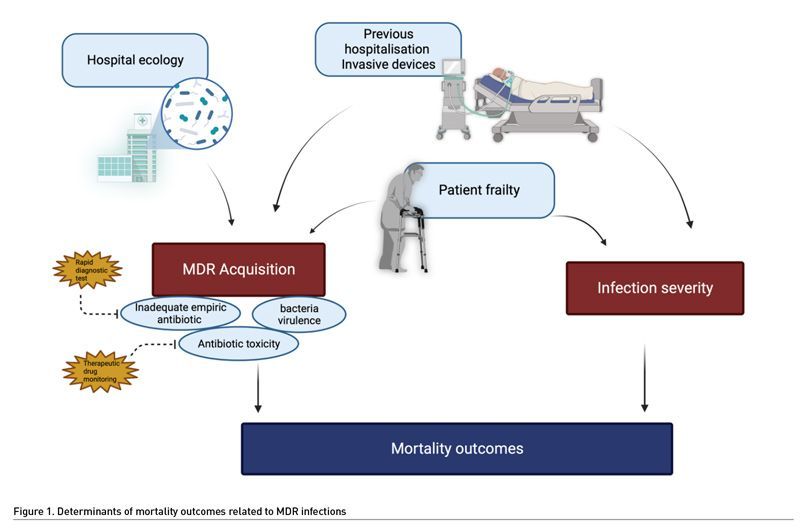
While the AMR burden on hospital length of stay and costs of hospitalisation is not a matter of debate, the relationship between AMR and hospital mortality rates remains controversial, particularly in the intensive care unit (ICU) setting. It is unclear if death should be attributed to the direct effect of AMR bacteria or the patient-related factors. Several studies found that the only independent predictor of hospital mortality was severity of sepsis, irrespective of the AMR status of the causative bacteria (Karvouniaris et al. 2022; Razazi et al. 2017). Lambert et al. (2011) published the largest prospective European ICU study (n=119,699 patients). They defined 20 different exposures according to infection site, pathogen, and resistance status, and then compared outcomes between patients exposed and unexposed. They found a modest, but significant, effect of AMR bacteria on the mortality rate. Risk of death associated with AMR bacteria was 1.2 (1.1-1.4) for pneumonia and 1.2 (0.9-1.5) for bloodstream infections. Interestingly, Pseudomonas aeruginosa had the highest burden of healthcare-acquired infections, independently of its resistance profile. Likewise, Paramythiotou et al. (2016) did not conclude a direct association between infections caused by resistant gram-negative bacteria and ICU mortality rates. One limitation is that most studies were conducted in single centres and included a small number of patients. In addition, they were characterised by a high degree of heterogeneity that prevented definitive conclusions from being made (Paramythiotou et al. 2016). Finally, the definitions of resistance are highly variable from one study to another.
A scoping review covering a broad time period from database inception to 2018 showed inconsistencies in AMR detection, AMR definitions and methods for measuring its attributable effect on outcomes (McDonald et al. 2021). On the contrary, three studies suggested that antibiotic resistance led to an increase in crude mortality, even after adjusting for two of them (Razazi et al. 2017; Bottazzi et al. 2018; Barbier et al. 2016). Furthermore, in a retrospective study based on a national database, our team found an association between ICU mortality and the occurrence of an infection due to AMR bacteria in case of ICU-acquired pneumonia (Lakbar et al. 2021). In this study, we assessed the association between pneumonia caused by highly AMR bacteria (including Staphylococcus aureus, Enterobacteriaceae, P. aeruginosa, or Acinetobacter baumanii) and ICU mortality on the whole sample and a 1:2 matched sample. We found that 3,081 (16.4%) out of 18,497 patients developed pneumonia due to highly AMR bacteria. The ICU mortality was higher in the patients infected with highly AMR bacteria than in those infected by non-highly AMR bacteria in the whole cohort (odds ratio (OR) 1.57 95% confidence interval (CI) [1.45-1.70], P < 0.001) and the matched cohort (OR 1.39 95% CI [1.27-1.52], P < 0.001). However, in this study, severity of patients was poorly detailed, which represents a bias for the assessment of outcomes. Indeed, examining the relationship between AMR and outcome is challenging, as it is difficult to discriminate the confounders and determinants of this relationship. Patients at the highest risk of death are also likely to be those at the highest risk of infection by AMR bacteria (Bottazzi et al. 2018). In addition, the ICU patients infected with AMR bacteria could be those in whom treatments may be limited or withdrawn because considered as futile, increasing per se their mortality rates.
At the bedside, AMR may affect the patient outcomes by three mechanisms. First, inadequate empirical antimicrobial therapy, i.e., giving antibiotics that are not efficient against the bacteria responsible for the current infection, is constantly associated with increased mortality (Retamar et al. 2012; Chen et al. 2013). In real life, the patients infected by AMR bacteria are at high risk to receive inadequate empirical treatment (Rottier et al. 2012). In addition, AMR can be associated with changes in pharmacokinetics, requiring for example higher doses of antibiotics (Mohd Sazlly et al. 2019). This may also explain failure to treat the patients infected by AMR. Second, bacterial virulence may be increased in AMR bacteria (Guillard et al. 2016). This hypothesis was tested in a murine model of infection due to P. aeruginosa (Roux et al. 2015). Acquisition of AMR resulted in improved fitness of the bacteria, promoting its survival and virulence. However, this hypothesis remains controversial since previous experimental models suggested a loss of virulence in multidrug resistant bacteria (Hraiech et al. 2013; Andersson and Hughes 2010). The third determinant is the patient themselves. The old, frail or immunosuppressed patients often required recurrent hospitalisations to conventional wards before ICU admission, exposure to repeated antibiotic treatments, and invasive procedures. Thus, they are at high risk of colonisation and/or infection by AMR bacteria (Giarratano et al. 2018). These patients are intrinsically at high risk of death, and infection by AMR bacteria could be considered as a symptom of their frailty. Finally, the effects of antibiotics themselves could be deleterious, as suggested previously (Jensen et al. 2011). A recent experimental study suggests that antibiotic exposure could result in a decreased immune response (Silva Lagos et al. 2022).
Antimicrobial Resistance During COVID-19 Pandemic
The COVID-19 pandemic seems to have potentiated the development of AMR bacteria. Reports from Europe and the U.S. suggested increasing AMR infection rates in the ICU during COVID-19 waves, especially due to ESKAPE multidrug resistant infections (Cogliati Dezza et al. 2022; Serapide et al. 2022; CDC 2022). In a systematic review and meta-analysis, Kariyawasam et al. (2022) identified 38 out of 1331 articles and found that prevalence of co-infection with resistant bacteria pathogens was 24% (95% CI 8-40%). Analyses suggested higher rates of AMR bacteria outside Europe and in ICUs. Among 58 (> 50%) non-survivors, all but six patients were infected with an AMR pathogen.
Of note, during the ICU stay, COVID-19 patients were at high risk of hospital-acquired infections (bloodstream and respiratory tract infections mostly) (Amarsy et al. 2022; Westblade et al. 2021) as they were subject to invasive devices, exposed to multiple antimicrobial treatments and potentially colonised with AMR bacteria. In addition, the inflammatory response they experienced exposed them to a risk of relative immunosuppression (Mehta et al. 2020; Vitte et al. 2020). Furthermore, the early stages of the pandemic were accompanied by the use of large amounts of antibiotics as prophylaxis while immunosuppressive therapy was an integral part of the therapeutic arsenal in COVID-19 patients. This certainly intensified the threat of antimicrobial resistance (Westblade et al. 2021; Rawson et al. 2020). Thus the surveillance systems should be maintained during pandemics, considering both the numerator and the denominator (Hirabayashi et al. 2021).
In our opinion, one of the lessons of this pandemic is that the principles of antimicrobial stewardship should be carefully studied in all situations. In addition, we need to improve our skills to accurately identify the profile of bacteria responsible for a bacterial infection, using rapid diagnostic tests to avoid hazardous empirical treatments.
Conclusion
In conclusion, AMR seems associated with increased ICU mortality rate, but the causality of this association remains unclear (Figure 1). Indeed, to our knowledge, no clinical study provided the required level of details making it impossible to discriminate the factors associated with the bacteria and those associated with the host. Whatever the nature of this association, this underlines the need to provide adequate antimicrobial therapy without delay, promoting the development of rapid diagnostic tests.
Conflict of Interest
None.
References:
Amarsy R, Trystram D, Cambau E et al.(2022) Surging bloodstream infections and antimicrobial resistance during the first wave of COVID-19: a study in a large multihospital institution in the Paris region. International Journal of Infectious Diseases. 114:90-96.
Andersson DI, Hughes D (2010) Antibiotic resistance and its cost: is it possible to reverse resistance? Nature reviews Microbiology. 8:260-271.
Barbier F, Pommier C, Essaied W et al.(2016) Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: What impact on outcomes and carbapenem exposure? Journal of Antimicrobial Chemotherapy. 71:1088-1097.
Bottazzi A, Chieregato A, Fumagalli R et al.(2018) Mortality attributable to different Klebsiella susceptibility patterns and to the coverage of empirical antibiotic therapy: a cohort study on patients admitted to the ICU with infection. Intensive Care Medicine. 44:1709-1719.
Cassini A, Högberg LD, Plachouras Det al. (2019) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. The Lancet Infectious Diseases. 19:56-66.
CDC (2022) COVID-19 & Antibiotic Resistance. Available from https://www.cdc.gov/drugresistance/covid19.html
CogliatiDezza F, Arcari G, Alessi F et al.(2022) Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Setting: Two Pandemics Compared. Antibiotics (Basel). 11:926.
Chen H-C, Lin W-L, Lin C-C et al.(2013) Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. Journal of Antimicrobial Chemotherapy. 68:947-953.
Giarratano A, Green S El, Nicolau DP (2018) Review of antimicrobial use and considerations in the elderly population. Clinical interventions in aging. 13:657-667.
Guillard T, Pons S, Roux D et al.(2016) Antibiotic resistance and virulence: Understanding the link and its consequences for prophylaxis and therapy. BioEssays : news and reviews in molecular, cellular and developmental biology. 38:682-693.
Hirabayashi A, Kajihara T, Yahara K et al.(2021) Impact of the COVID-19 pandemic on the surveillance of antimicrobial resistance. J Hosp Infect. 117:147-156.
Hraiech S, Roch A, Lepidi H et al.(2013) Impaired virulence and fitness of a colistin-resistant clinical isolate of Acinetobacter baumannii in a rat model of pneumonia. Antimicrobial agents and chemotherapy. 57:5120-5121.
Jensen JU, Hein L, Lundgren B et al.(2011) Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial. Critical Care Medicine. 39:2048-2058.
Kariyawasam RM, Julien DA, Jelinski DC et al.(2022) Antimicrobial resistance (AMR) in COVID-19 patients: a systematic review and meta-analysis (November 2019-June 2021). Antimicrob Resist Infect Control. 11:45.
Karvouniaris M, Poulakou G, Tsiakos K et al.(2022) ICU-Associated Gram-Negative Bloodstream Infection: Risk Factors Affecting the Outcome Following the Emergence of Colistin-Resistant Isolates in a Regional Greek Hospital. Antibiotics. 11:405.
Lakbar I, Medam S, Ronflé R et al.(2021) Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci Rep. 11:16497.
Lambert ML, Suetens C, Savey A et al.(2011) Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: A cohort study. The Lancet Infectious Diseases. 11:30-38.
McDonald KL, Garland S, Carson CA et al.(2021) Measures used to assess the burden of ESBL-producing Escherichia coli infections in humans: a scoping review. JAC-Antimicrobial Resistance. 3:dlaa104.
Mehta P, McAuley DF, Brown M et al.(2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 395:1033-1034.
MohdSazlly Lim S, Sime FB, Roberts JA (2019) Multidrug-resistant Acinetobacter baumannii infections: Current evidence on treatment options and the role of pharmacokinetics/pharmacodynamics in dose optimisation. International Journal of Antimicrobial Agents. 53:726-745.
Murray CJ, Ikuta KS, Sharara F et al.(2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 399:629-655.
Paramythiotou E, Routsi C (2016) Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World Journal of Critical Care Medicine. 5:111.
Rawson TM, Moore LSP, Castro-Sanchez E et al.(2020) COVID-19 and the potential long-term impact on antimicrobial resistance. Journal of Antimicrobial Chemotherapy. 75:1681-1684.
Razazi K, MekontsoDessap A, Carteaux G et al.(2017) Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum β-lactamase-producing Enterobacteriaceae. Annals of Intensive Care. 7:1-7.
Retamar P, Portillo MM, López-Prieto MD et al.(2012) Impact of Inadequate Empirical Therapy on the Mortality of Patients with Bloodstream Infections: a Propensity Score-Based Analysis. Antimicrob Agents Chemother. 56:472-478.
Rottier WC, Ammerlaan HSM, Bonten MJM (2012) Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing enterobacteriaceae and patient outcome: A meta-analysis. Journal of Antimicrobial Chemotherapy. 67:1311-1320.
Roux D, Danilchanka O, Guillard T et al.(2015) Fitness cost of antibiotic susceptibility during bacterial infection. Science Translational Medicine. 7.
Serapide F, Quirino A, Scaglione V et al.(2022) Is the Pendulum of Antimicrobial Drug Resistance Swinging Back after COVID-19? Microorganisms. 10:957.
Silva Lagos L, Luu TV, De Haan B et al.(2022) TLR2 and TLR4 activity in monocytes and macrophages after exposure to amoxicillin, ciprofloxacin, doxycycline and erythromycin. Journal of Antimicrobial Chemotherapy. dkac254.
Vitte J, Diallo AB, Boumaza A et al. (2020) A Granulocytic
Signature Identifies COVID-19 and Its Severity. J Infect Dis.
13;222(12):1985-1996.
Westblade LF, Simon MS, Satlin MJ (2021) Bacterial Coinfections in Coronavirus Disease 2019. Trends in Microbiology. 29:930-941.
WHO (2015) Global action plan on antimicrobial resistance. Geneva: World Health Organization. Available from https://apps.who.int/iris/handle/10665/354612