HealthManagement, Volume 14 - Issue 4, 2014
The transmission of pathogens through blood and other body fluids represents one of the main risks in the healthcare setting. Although over 20 pathogens can be transmitted in this way, the most important are the hepatitis B (HBV) and C (HCV) viruses and the AIDS virus (HIV).
Since 2006 it has been mandatory in the Madrid Region of Spain to use safety-engineered devices in all healthcare institutions and, on 11 May 2013, the European Directive on the prevention of sharp injuries came into effect. The Directive focuses on two main aspects:
- Healthcare worker safety and health are essential and are intimately linked to
patient health and safety.
- The key and strategic element of the Directive is the minimisation of exposure
risk and reducing the number of injuries, but not the number of infections as
a result of exposure.
In our experience, there are several key issues for ensuring the effective implementation of safety-engineered devices. The first is of a strategic nature and consists of the promotion from the Hospital Management of a specific safety culture through the adoption of a general prevention policy encompassing all the workers at risk and all the processes, in which sharp objects are used. This, in turn, inevitably leads us to another aspect, namely the adoption of a method or tool for knowing and evaluating all the processes, in which sharp objects intervene. It is very important to modify the traditional management system and introduce process management. In a very schematic manner, this model offers added value that allows us to integrate and align processes in order to obtain the planned results. Furthermore, it focuses effort on the efficacy and efficiency of processes and fosters operational transparency within the organisation. Lastly, it reduces cycle times and costs through efficient resource use.
A second key issue is identifying the risk perception of the professionals, their sensitivity to such risk, and their adherence to a new biosafety culture. The greatest infection risk following a percutaneous accident corresponds to hepatitis B (30%), followed by hepatitis C (3%) and HIV infection (0.3%). Such accidents pose a serious occupational health problem due to their high frequency, potential seriousness and associated costs. In this regard, percutaneous accidents represent the most important transmission route. The risk of transmission following exposure clearly depends on the source patient characteristics, the type of exposure, and the serological status of the exposed person (level of evidence II).
Risk is highest when exposure involves extensive contact with blood through deep punctures or cuts with contaminated hollow needles that had previously been located in an artery or vein (level of evidence III). According to the latest data of our hospital (2014), 68% of all accidents involved hollow needles. Of these, 72% corresponded to small-calibre needles. It is interesting to examine the materials affected after 8 years of biosafety measures. In this respect, subcutaneous, intramuscular and butterfly needles were responsible for 25.7%, 18.5% and 9% of the injuries, respectively, while intravenous catheters accounted for 8.7%, intradermal needles 5.6%, blood gas syringes 3.7%, arterial catheters 0.3%, and central venous catheters 0.3%. This profile is clearly different from that which can be found in a healthcare centre lacking biosafety measures, and, although the results require more exhaustive analysis, we do know that we must not neglect processes such as drug aspiration and injection versus infusion and extraction, as evidenced by our own experience.
Another very important aspect is the adherence by the healthcare professional to the biosafety device and biosafety culture. In this context, the professional should actively participate in the choice of devices through qualitative assessment of the products, once the Occupational Risk Prevention Department in coordination with the Material Resources Unit has determined the technical characteristics after creating the pertinent work groups.
A third key issue refers to effective implementation of the devices. Before deciding on a massive purchase, we recommend conducting a pilot test in sensitised units, with the participation of workers affected in the use of the device, because it must be tested in real-life situations. This will provide very useful information and can serve to identify potential problems.
Once the devices have been chosen, the workers must be trained to use them. In this regard, caution is required to choose devices according to the specific unit in which they are to be used. At the time of actual implementation, no devices with and without safety-engineered features measures for one same procedure should coexist, and systematisation is moreover needed, with the adoption of a logical order. We recommend an analysis to compile data on the frequency of accidents, and the use of a Pareto diagram to know where 80% of the accidents have occurred – thereby, allowing us to establish priorities and ensure effective implementation and investment.
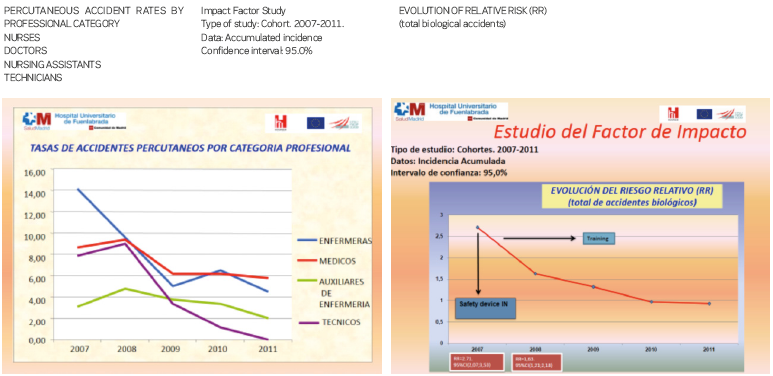
In a fourth stage, we must evaluate the impact factor of the implementation of the devices, answering two crucial questions: Are they effective? How many accidents do we prevent? According to a study carried out in our hospital between 2007 and 2011, we obtained the following results:
- Adoption of the safety-engineered devices reduced the risk of accidents (DAR
-0.45; 95%CI -0.06 to -0.02; p<0.05). Similar conclusions about the efficacy
of such devices were drawn by De Carli from the SIROH study between 1997-2010.
- One accident for every 22 professionals was avoided (NNT 22.2; CI 15.4 – 37.7;
p<0.05)
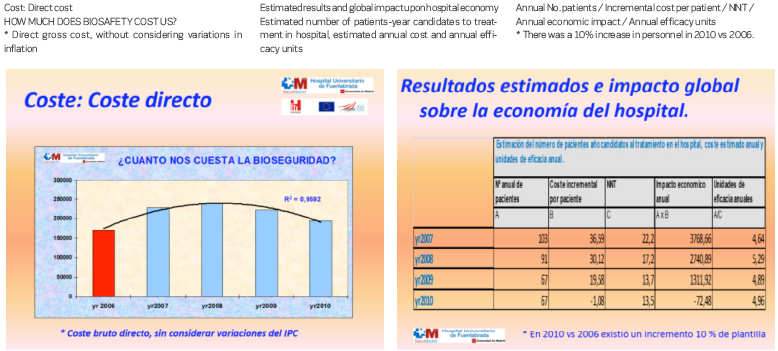
Finally, due to its importance, a cost-efficacy analysis must be carried out. In our study we did not consider estimating the binomial direct – indirect costs. Rather, we aimed to identify the cost of the resources needed to reduce an adverse effect, taking only the product cost into account (analysing the actual product cost and the incremental cost-effectiveness). Clearly, the prices of the devices have decreased considerably with respect to the year 2007 – a fact that favoured our analysis. The results obtained indicate that amortisation of the initial investment is achieved within four years.
A final, but not less important comment, refers to the legal responsibilities and consequences in the European Union, resulting from the seroconversion of an individual secondary to inoculation, considering the existence of a Specific Directive and the availability of safety-engineered devices products on the market.















