ICU Management & Practice, Volume 21 - Issue 5, 2021
Clostridioides difficile (C. difficile) infection is a potentially serious complication in critical patients admitted to the Intensive Care Unit (ICU). It generally occurs because of an alteration of the intestinal microbiota due to antibiotic exposure that must be timely identified and diagnosed to start proper and early management.
Pathophysiology
The digestive tract extends from the mouth to the rectum. Its covering mucosa, with an approximate 300 m2 surface, acts as a barrier against microbial invasion, mainly through three levels of control: first, gastric acid is responsible for eradicating ingested microorganisms; secondly, the mucosa, which has a single layer of columnar epithelial cells (0.1 mm thick), acts as a physical barrier, blocking the bacteria and toxins movement into circulation; and, finally, the reticuloendothelial system traps and destroys the microorganisms that cross the mucosa (Martínez-Rodríguez et al. 2018).
The gastrointestinal tract is widely colonised, being the large intestine the most populated region, which reaches up to 1012 bacteria per gram of fecal matter (1-1.5 kg per weight). Knowing this fact has allowed us to understand the important protective role that this intraluminal ecosystem plays, which prevents invasion by pathogenic microbes capable of causing disease. The effect of antibiotics on the intestinal microbiota is well documented. These show a long-term reduction in bacterial diversity after their use, which decreases resistance to colonisation. Furthermore, this microbiota modification after antibiotic treatment facilitates the transfer of drug-resistance genes (Portillo et al. 2002; Meyer et al. 2014).
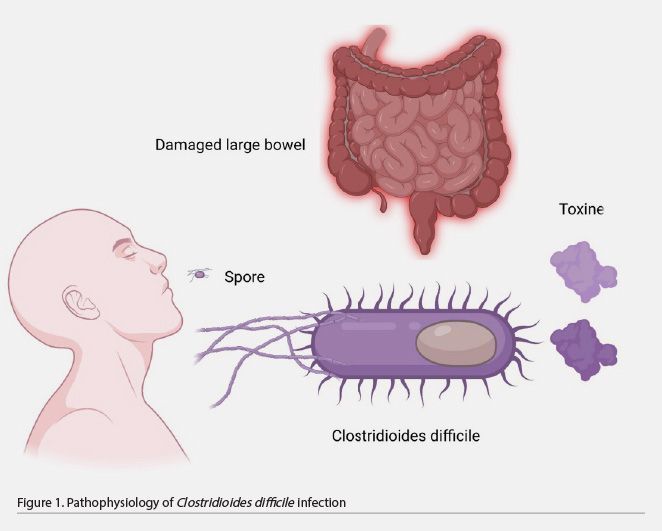
C. difficile is a bacterium that forms acid-, antibiotic-, and heat-resistant spores that spread through fomites or directly by the oral-fecal route. The bacillus does not survive gastric acid; however, the spores are resistant to its effects and germinate when exposed to bile salts in the small intestine. These spores later colonise the large intestine with bacilli, causing disease in susceptible people. The use of antibiotics is the main associated factor. At this site, it acts by releasing two protein exotoxins, toxins A and B, whose effects lead to pseudomembranes or even megacolon formation (Table 1 and Figure 1) (Meyer et al. 2014; Barra-Carrasco et al. 2014).
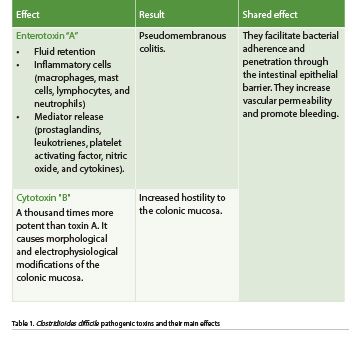
The following are characteristics of C. difficile-induced pseudomembranous colitis.
- Early or type I lesion: the patchy necrosis of the epithelium forms fibrin and fibrinous exudate in the lumen of the colon.
- The exudative lesion, or type II lesion, is a volcano-type epithelial ulceration with intact surrounding mucosa.
- Type III lesion: diffuse epithelial necrosis and ulceration with development of a pseudomembrane containing cellular debris, leukocytes, fibrin, and mucin.
C. difficile initiates a sporulation process that consists of producing spores that are spread into the environment in stools, a unique and sophisticated strategy to persist in the colonic environment of the host. This occurs when environmental conditions are unfavourable for its survival (Portillo et al. 2002).
Risk Factors
Risk factors for CDI include being 65 years or older, previous hospitalisation, recent antimicrobial therapy (particularly third-generation cephalosporins, amoxicillin-clavulanate, clindamycin, and newer fluoroquinolones), immunosuppression, and proton pump inhibitors.
Likewise, there are factors associated with the patient themselves, related to advanced age, such as chronic diseases and multiple comorbidities of which inflammatory bowel disease, chronic liver disease, and immunosuppression stand out (De Roo and Regenbogen 2020).
Clinical Conditions
The CDI clinical conditions are highly heterogeneous, ranging from asymptomatic carrier status, mild to moderate diarrhoea, to life-threatening fulminant colitis. Although the incubation period is not precisely described, some reports suggest it lasts from 2 to 3 days, but more recent studies show that it can be longer than 3 days, and it depends on each individual. The CDI can affect all parts of the colon, but the distal segment is the most commonly infiltrated. Most patients with CDI have mild diarrhoea and experience spontaneous recovery after 5-10 days of completing the course of antibiotics (Samore et al. 1994; McDonald et al. 2018).
To make an effective diagnosis of CDI, both clinical symptoms and a positive lab-test result are required (Zhong et al. 2018). The clinical condition ranges from mild diarrhoea to severe illness or fulminant colitis. Up to 30% of patients can develop a recurrent CDI. Although diarrhoea is the characteristic symptom, it may not be present at the onset of the disease, possibly due to colon dysmotility, either from previous underlying conditions or from the disease process (Sartelli et al. 2019).
Mild to Moderate CDI
Diarrhoea is defined as loose stools corresponding to types 5-7 of the Bristol Stool Chart. The patient must present at least three diarrhoeal stools for 24 consecutive hours or more frequently than normal for the patient. Diarrhoea must be accompanied by mild abdominal pain and cramps. If prolonged, it can cause an alteration of the water and electrolyte balance as well as dehydration (Zhong et al. 2018).
Severe CDI
Severe CDI is associated with increased abdominal pain and cramps, as well as systemic symptoms such as fever, leukocytosis, and hypoalbuminaemia. The absence of diarrhoea may indicate the progression of fulminant disease. Although a wide variety of predictors of poor prognosis have been described, there is still no international consensus for the severe CDI definition. Progression to fulminant colitis is relatively uncommon (1-3% of all CDIs). Mortality remains high due to the development of toxic megacolon with colonic perforation, peritonitis, septic shock, and subsequent organ dysfunction (Sartelli et al. 2019).
Severity markers include advanced age (≥65 years), leukocytosis (> 15 × 109/L), lower blood albumin levels (< 2.5 g/dL), elevated serum creatinine levels (≥ 133 µM or ≥ 1.5 times the baseline), temperature > 38.5, severe underlying disease or previous immunodeficiency (Zhong et al. 2019). In a recent study, it was shown that human serum albumin is capable of binding to the IIa domain of toxins A and B of C. difficile, which prevents its internalisation in host cells. This could partially explain the hypoalbuminaemia with a CDI severity marker.
Recurrent CDI
In 10-30% of cases, a recurrence of symptoms develops after initial therapy for C. difficile and it becomes a clinical challenge. For a patient who has presented 1 to 2 cases, the risk of more recurrences is 40-65%. Recurrent CDI may result either from the germination of resident spores that remain in the colon after completing the antibiotic treatment or from reinfection from an environmental source. Recurrence is present when the CDI reappears within 8 weeks of the onset of a previous episode and after its symptoms resolve once the initial treatment is completed. In daily practice, it is difficult to distinguish between recurrence due to relapse or reinfection (Di Masi et al. 2018).
When a patient has diarrhoeal stools that correspond to Bristol stool types 5-7 and has other CDI risk factors together with the absence of a different cause of diarrhoea, a stool sample should be collected for laboratory analysis. However, for paralytic ileus, formed stool samples should not be tested for CDI (Di Masi et al. 2018).
Diagnosis
Only toxigenic strains, which produce toxins A and B, are pathogenic. According to the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) guidelines, CDI is defined as a condition compatible with CDI plus microbiological evidence of toxins A and B that produce C. difficile in stool, without evidence of another cause of diarrhoea, or patients with pseudomembranous colitis (Di Masi et al. 2018).
Currently, there is no single stool test to be used as a standalone test for diagnosing CDI. Several laboratory tests detect free toxins in the stool (enzyme immunoassay (EIA), cell cytotoxicity neutralisation assay (CCNA), C. difficile presence (EIAs that detect glutamate dehydrogenase (GDH)), or the presence of a toxigenic C. difficile strain (toxigenic culture (TC)), and nucleic acid amplification tests (NAAT) (Di Masi et al. 2018).
Among these methods, stool TC or CCNA have been considered the gold standard for the diagnosis of CDI for the past 30 years. Paradoxically, neither of these are used routinely due to technical problems and the prolonged time of results (Di Masi et al. 2018).
1. Toxicogenic culture (TC) is a two-step method that first isolates C. difficile strains on a selective medium, and then evaluates in vitro toxin-producing capacity. Different selective media are available and are generally derived from cycloserine-cefoxitin fructose agar. Currently, additives such as sodium taurocholate or lysozyme have been added to stimulate germination. Chromogenic media have also been developed since it has been shown that they are as sensitive as other selective media, which allows identification within 24 hours after incubation. Plates are incubated in an anaerobic atmosphere for 48 hours at 36 ± 1 °C. After isolating a strain, its pathogenic potential is determined by testing for in vitro toxin production. TC is considered the gold standard for detecting toxigenic C. difficile and for evaluating new molecular methods. Although TC results take too long for routine diagnosis (2 to 5 days), culture is essential for subsequent strain typing, molecular analysis, and antimicrobial susceptibility determination (Di Masi et al. 2018; Crobach et al. 2016).
2. Nucleic Acid Amplification Test (NAAT) for C. difficile: C. Difficile toxin genes were introduced in 2009. NAATs are based on a PCR method or isothermal amplification. They have a higher sensitivity (80-100%) and specificity (87-99%) than EIA tests, so they can be used as a CDI standard diagnostic test. NAAT, as a one-step algorithm, can increase the detection of asymptomatic colonisation; therefore, it should be performed in patients with high suspicion of CDI, or included in a two-step algorithm starting with toxin detection. This test has limitations such as its high cost and some difficulties in its interpretation. PCR detects the presence of a toxin-encoding gene, thus confirming the presence of toxin-producing C. difficile, but this does not necessarily mean that the strain is producing toxins at that time, resulting in false positives (Crobach et al. 2016).
3. Glutamate dehydrogenase (GDH) tests: Glutamate dehydrogenase is a metabolic enzyme expressed in all C. difficile strains. A positive result only indicates the presence of C. difficile, without predicting the strain’s ability to produce toxins. GCH can be detected by immunoenzymatic assays (ELISA) or immunochromatography. At present, different guidelines propose GDH EIA tests as a detection method for diagnosing CDI. Due to its high negative predictive value (NPV) of 80-100%, a negative test will rule out infection. However, a positive result must be confirmed by a second, more specific test that detects toxins (Di Masi et al. 2018; Crobach et al. 2016).
4. Toxin A/B enzyme immunoassay (EIA): EIA is a fast test that provides results in about 1 to 2 hours, and has a 75-85% sensitivity and a 95%-100% specificity. Due to its low cost and ease, it is the most popular in laboratories. However, many studies have highlighted its lack of sensitivity (ranging from 29% to 86%) in comparison to CCNC, which excludes its use as a stand-alone test for the diagnosis of CDI (Crobach et al. 2016).
5. Cell culture cytotoxicity neutralisation assay (CCNA): It is considered the gold standard for detecting free toxins (mainly toxin B) in stools. For this method, stool filtrates are inoculated onto a cell culture which is then observed for a cytopathic effect evaluated at 36 ± 1 °C after 1 or 2 days. The specificity of the cytopathic effect is evaluated by the neutralisation with C. difficile antitoxin or Clostridium sordelli antitoxin sera, which share the same antigens. Despite CCNA’s good sensitivity, specificity, and low cost, this method is currently used by a very limited number of laboratories due to the lack of standardisation and prolonged response time (Di Masi et al. 2018).
According to the ESCMID, no test is suitable as a stand-alone test for diagnosing CDI since they have a low positive predictive value. The best way to optimise the CDI diagnosis is by combining two tests in a two-step algorithm (Figure 2). The first test should be a high negative predictive value test (GDH or NAAT). The second test should be a high positive predictive value test (toxin A/B EIA). If the first test is negative, DCI is excluded; if it is positive, a second test should be performed to confirm the diagnosis. If the second test is positive, the CDI diagnosis is confirmed; if it is negative, the case must be clinically evaluated. In this scenario, the possible cause can be related to 3 situations: CDI with toxin levels below the threshold of detection, false-negative result, or C. difficile carriage (Figure 2) (Crobach et al. 2016).
Proper handling in the preanalytical phase is extremely important as it can lead to wrong results. The toxin present in a stool sample breaks down easily at room temperature and can no longer be detected after 2 hours. Thus, when the sample is obtained it should be stored at 4 °C temperature and the test must be performed within the next 24 hours. The test should only be performed on a diarrhoeal stool sample. If the patient has ileus, a rectal swab can be used. Tests in asymptomatic patients are not recommended, unless for epidemiological purposes. Repeat testing for C. difficile after successful completion of treatment is also not recommended, as some patients may have positive results without requiring continued or repeat treatment (Czepiel et al. 2019).

Treatment
Once a CDI diagnosis is confirmed and if the patient is symptomatic, the first step is to stop all antimicrobials. The selection of antibiotics should be based on the severity criteria, considering whether it is the first occurrence or a recurrence. For mild to moderate initial infection, treatment with oral vancomycin at a 125 mg QID dose for 10 days is recommended (Abreu et al. 2019). Treatment with fidaxomicin (a narrow-spectrum antibiotic of specific antibacterial activity due to its inhibition of bacterial RNA polymerase) at a 200 mg BID dose for 10 days is an alternative to vancomycin. Recent clinical trials have shown the non-inferiority of fidaxomicin compared to vancomycin; it even has a lower recurrence rate than vancomycin (Polivkovaa et al. 2021). If vancomycin or fidaxomicin are not available, it is recommended to use metronidazole as an alternative treatment, which is prescribed at a 500 mg TID dose for 10 days. In patients who cannot tolerate the oral route, this antibiotic can be administered intravenously. The lack of response to metronidazole after 5 days of treatment is an indication for a change from the antibiotic to oral vancomycin at a 125 mg QID dose for 10 days (Abreu et al. 2019; Johnson et al. 2021; Antonelli et al. 2020).
In severe complicated CDI, combination treatment of oral vancomycin at 250 to 500 mg QID doses combined with metronidazole 500 mg TID intravenously for 14 days is the treatment of choice. In severe-complicated cases with abdominal distention or ileus, it is recommended to administer vancomycin at a 500 mg QID dose via a rectal tube (Abreu et al. 2019; Johnson et al. 2021; Antonelli et al. 2020).
For patients with multiple recurrences, vancomycin is recommended at a 125mg QID dose for 10 to 14 days, followed by rifaximin 400 mg TID for 20 days or fidaxomicin 200 mg BID for 10 days (Abreu et al. 2019; Polivkovaa et al. 2021).
Patients with a septic shock, or multiple organ failure (with clinical evidence of toxic megacolon, peritonitis, or perforation), who have failed treatment are candidates for surgical intervention. Surgery should also be considered in patients with severe CDI who do not respond to antibiotic treatment. The surgical intervention of choice is subtotal colectomy with terminal ileostomy since segmental colectomies have a worse prognosis (Figure 3) (Sartelli et al. 2019).
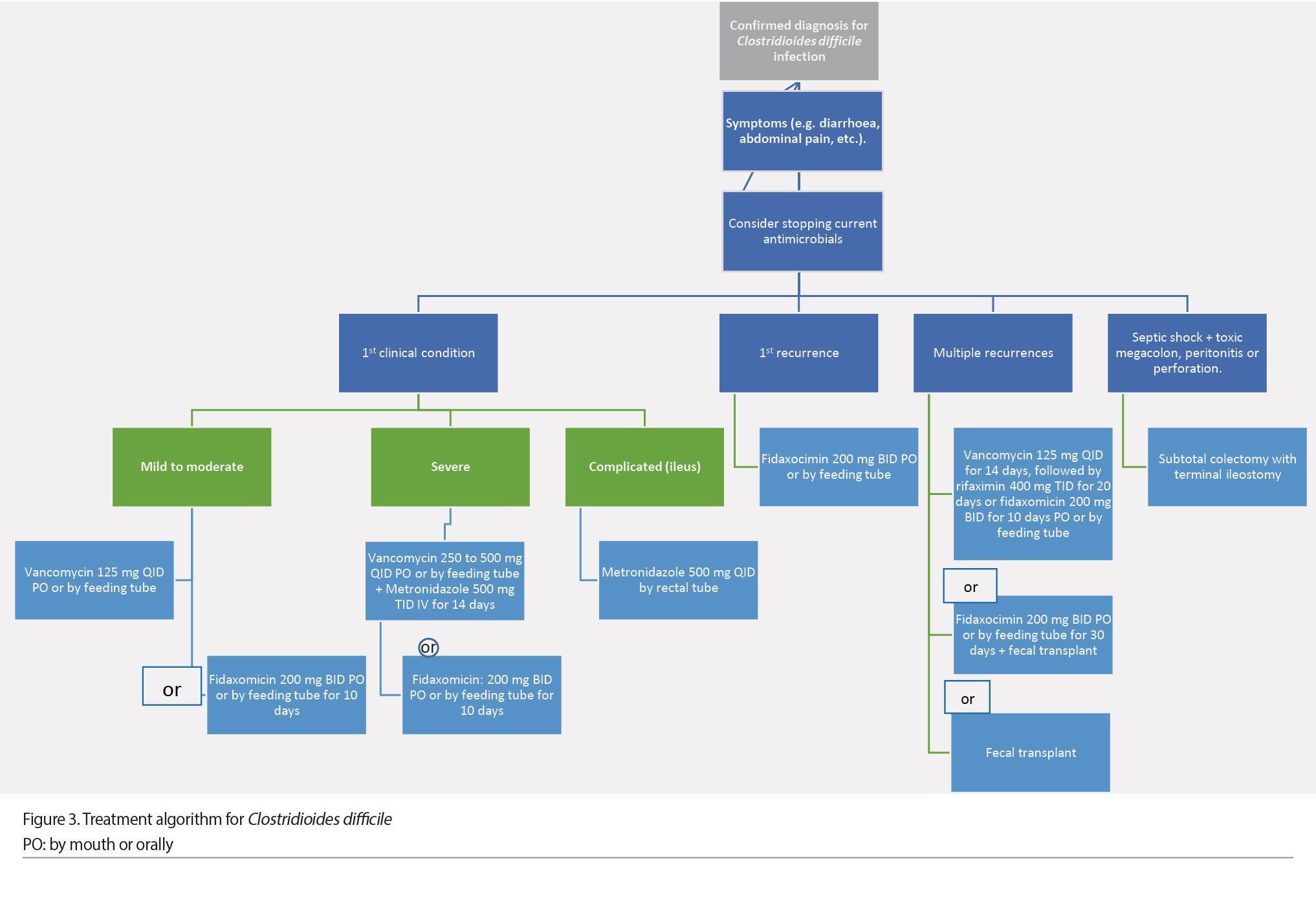
Fecal microbiota transplant (FMT) is a safe and effective option in CDI patients with two recurrences or severe episodes, when antimicrobial treatment fails. It consists of the infusion of stool (containing the entire community of the intestinal microbiota) from a healthy donor to the digestive tract of the patient to cure or improve a disease. The purpose of FMT in CDI treatment is to restore the diversity of microorganisms in the colonic microbiota and to stop C. difficile growth.
FMT is indicated for the following cases:
• Recurrent C. difficile infection.
- 3 or more episodes of mild to moderate CDI (1 initial and 2 recurrences) when treatment with vancomycin for 6 to 8 weeks fails, whether combined with another alternative antibiotic (fidaxomicin, rifaximin, nitazoxanide) or not.
- 2 or more episodes of CDI with hospital admission and significant morbidity.
The microbiota donor can be a known donor (family member, friend, spouse) or a universal donor (anonymous). The donor must be a healthy subject without digestive or extradigestive comorbidities, and risk of transmitting an infectious agent, and have not used antibiotics in the last three months. Routes of FMT administration include nasogastric tube, colonoscopy, enema, or capsule, which all have been shown effective (Abreu et al. 2019; Chun-Wei et al. 2021).
Bezlotoxumab can be used as a co-intervention with antibiotics for patients with a recurrent CDI in the past 6 months to reduce the risk of a subsequent CDI recurrence after initial clinical recovery. In patients with a history of congestive heart failure, the US Food and Drug Administration (FDA) advises that bezlotoxumab should be reserved for use only when the benefit outweighs the risk. There are comparative trials of different anti-CDI recurrence strategies using narrow-spectrum antibiotics that target C. difficile, restoration of the microbiota by biotherapeutics or FMT, or increase of host immune response with single-administered agents, such as bezlotoxumab (Johnson et al. 2021).
In conclusion, C. difficile infection is a serious disease that must be appropriately recognised and treated due to its high risk of spread and its potentially serious complications. Therefore, avoiding the indiscriminate use of antibiotics for hospitalised patients is crucial.
Conflict of Interest
None.
References:
Abreu AT, Abreua JA, Velarde-Ruiz V et al. (2019) Consenso sobre prevención, diagnóstico y tratamiento de la infección por Clostridium difficile, Revista de Gastroenterología de México, 84(2):204-219.
Antonelli M, Martin-Loeches I, Dimopoulos G et al. (2020) Clostridioides difficile (formerly Clostridium difficile) infection in the critically ill: an expert statement; Intensive Care Med.
Chun-Wei Chiu, Pei-Jane Tsai, Ching-Chi Lee et al. (2021)Application of Microbiome Management in Therapy for Clostridioides difficile Infections: From Fecal Microbiota Transplantation to Probiotics to Microbiota-Preserving Antimicrobial Agents; Pathogens. 10:649.
Crobach, MJT, Planche T, Eckert C (2016) European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clinical Microbiology and Infection, 22(4):S63eS81.
Czepiel J, Dróżdż M, Pituch H et al. (2019) Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis, 38(7):1211-1221.
Di Masi A, Leboffe L, Polticelli F et al. (2018) Human serum albumin is an essential component of the host defense mechanism against Clostridium difficile intoxication. J Infect Dis, 22:1424–35.
De Roo AC, Regenbogen SE (2020) Clostridium difficile Infection: An Epidemiology Update. Clinics in Colon and Rectal Surgery, 2(33):49-57.
Johnson S, Lavergne V, Skinner AM et al. (2021) Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Infectious Diseases Society of America Guidelines.
Jonathan Barra-Carrasco, Cristian Hernández-Rocha, Patricio Ibáñez et al. (2014) Clostridium difficile spores and its relevance in the persistence and transmission of the infection. Rev Chilena Infectol, 31(6):694-703.
Martínez-Rodríguez AA, Estrada-Hernández LO, Tomé-Sandoval P, Salazar-Salinas J (2018) Diarrea por Clostridium difficile en pacientes hospitalizados. Med Int Méx, 34(1):9-18.
McDonald LC, Gerding DN, Johnson S et al. (2018) Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis, 66:e1–e48.
Meyer SL, Espinoza AR, Quera PR (2014) Clostridium difficile infection: epidemiology, diagnostic and therapeutic strategies. Rev. Med, 25(3):473-484.
Polivkovaa S, Krutovab M, Capek V et al. (2021) Fidaxomicin versus metronidazole, vancomycin and their combination for initial episode, first recurrence and severe Clostridioides difficile infection - An observational cohort study, International Journal of Infectious Diseases, 226–233.
Portillo LM, Castellanos-UAC, Nava CE, Chiprut R (2002) Infección por Clostridium difficile. Gaceta Médica, 138 No. 1.
Samore MH, DeGirolami PC, Tlucko A et al. (1994) Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis, 18(181):187 47.
Sartelli M, Di Bella S, McFarland LV et al. (2019) Update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg, 28;14:8.
Peng Z, Ling L, Stratton CW et al. (2018) Advances in the diagnosis and treatment of Clostridium difficile infections. Emerging Microbes & Infections, 7:15.


























