Tackling Attrition: British Medical Associati
- EXEC
- 23/04/2024
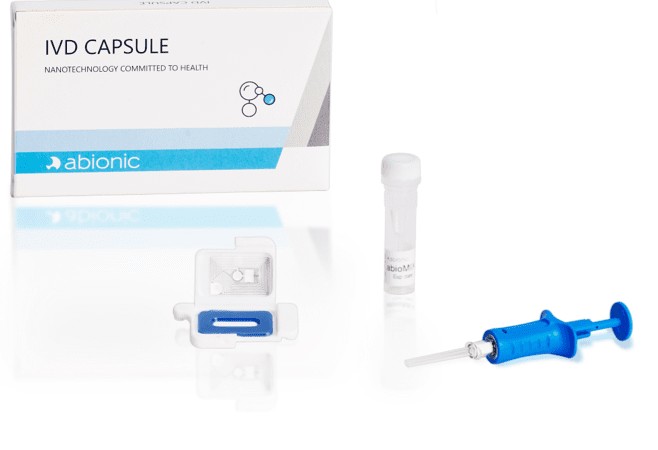
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in adults.
Pancreatic Stone Protein (PSP) on the abioSCOPE® is the Earliest Marker of Sepsis
Sepsis is a major healthcare burden claiming more than 11 million lives per year, one death every 2.8 seconds1. It is caused by a dysregulated host response to infection which can progress to multiple organ dysfunction, septic shock and death. It’s a medical emergency that requires immediate diagnosis. Unfortunately, current standards of care often lead to sepsis being diagnosed too late.
The clinical signs and symptoms of sepsis are generic and non-specific, making it extremely challenging to timely identify. The availability of an early and accurate biomarker at the patient’s bedside is key to enabling faster treatment decisions, reduce mortality and lower sepsis-related healthcare costs.
EARLY SEPSIS DETECTION UP TO 72 HOURS BEFORE THE STANDARD OF CARE IN 5 MIN*
The PSP Test on the abioSCOPE® Can Save Millions of Lives
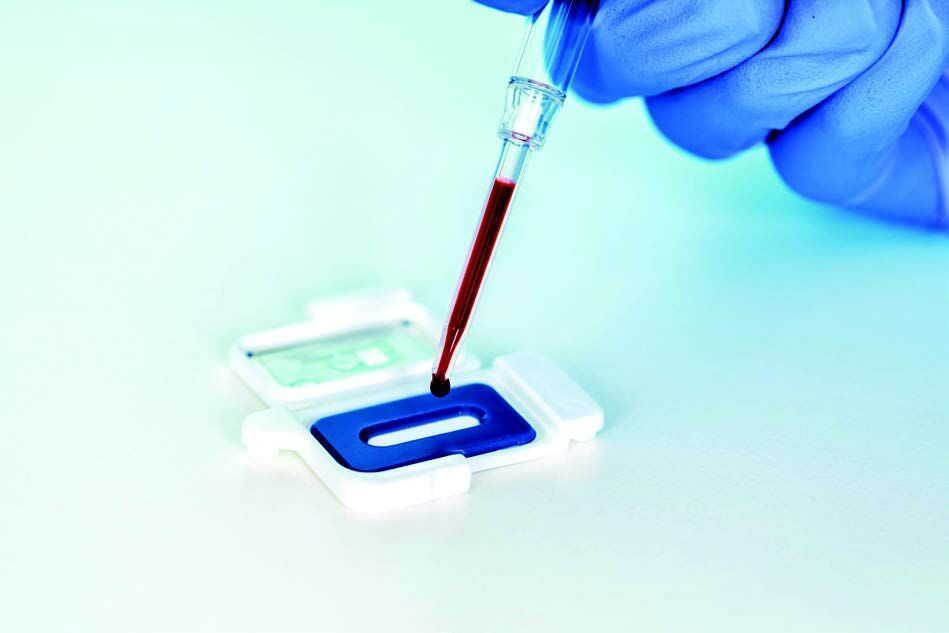
The IVD CAPSULE PSP on the abioSCOPE® is the first CE-marked in vitro diagnostic test to enable fast, reliable and early sepsis detection at the point-of-care from a single drop of blood in only 5 minutes*.
A multicentric study published recently in Critical Care, proves that bedside measurement of PSP on the abioSCOPE® clearly correlates with the onset of sepsis, enabling personalized clinical management of patients in the ICU3.
An increasing PSP concentration in the days preceding the clinical diagnosis of sepsis, offers a unique window of opportunity for clinicians to initiate timely the right treatment.
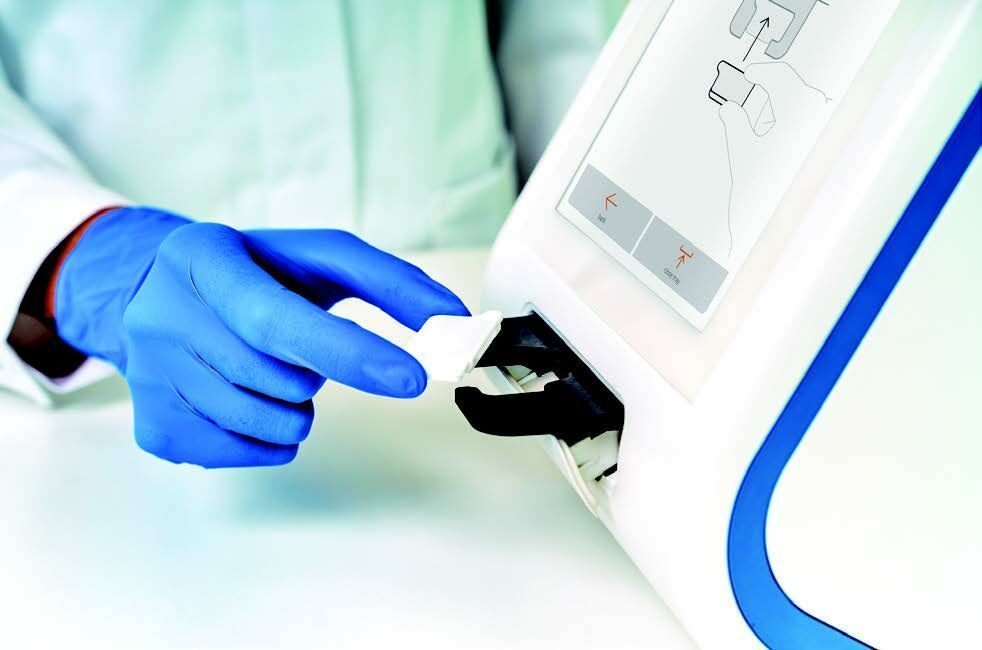
IVD CAPSULE PSP ON THE ABIOSCOPE DESIGNED FOR ON-DEMAND USE IN THE ICU
Scope of Use for the PSP Biomarker in the Diagnosis of Sepsis in Adults
Compact, robust, and intuitive to use, Abionic’s PSP test on the abioSCOPE® is fully compatible with hospital information systems and fits seamlessly into the workflow. When sepsis is suspected, an immediate access to reliable test results is essential. Serial bedside PSP
measurements every 24 hours can aid the clinical management and early identification of sepsis in patients at risk. A constantly low PSP value is also a strong indicator of a patient's stability, supporting the decision to not start or withhold unnecessary antibacterial treatment.
References:
* IVD CAPSULE PSP on the abioSCOPE® measurment time: 5 minutes; Total Assay time: 7.5 minutes.
Key Fatures:
Download PSP Product Leaflet: https://www.abionic.com/sites/default/files/pdf/D4haab_210610_PSP_Brochure_JGA.pdf
Loading...