Can GPT-4 Enhance Radiology Workflows by...
- Imaging
- 18/04/2024
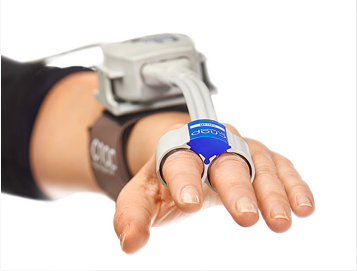

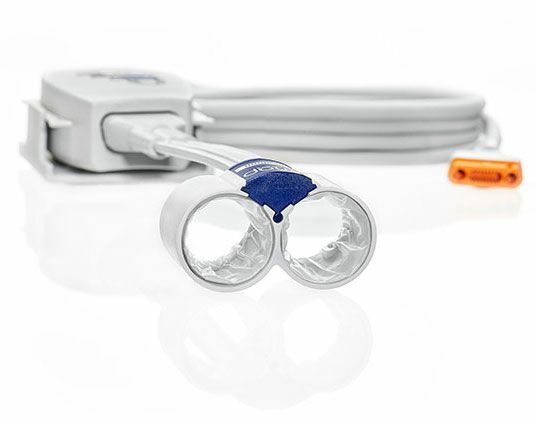



The CNAP® OEM Module fully integrates into your host device with an extensive protocol, power supply and NBP calibration are provided by the host. This customizable solution comes with a Developer Implementation Kit and CNSystems integration and regulatory support.
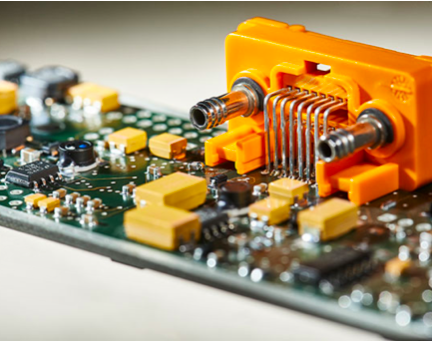
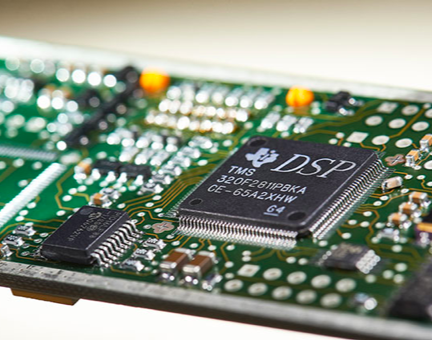
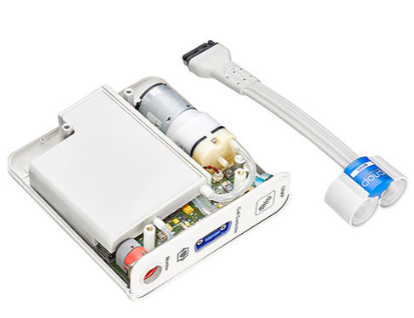
Our Task Force® CORE platform allows for efficient digital integration into your host software using a USB driver and simplifies protocol or easily interfaces with up to eight isolated analog channels.


CNSystems provides the following services to support a successful integration:

Loading...









