ICU Management & Practice, Volume 16 - Issue 3, 2016
Knowledge of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) is crucial for successful treatment of critically ill patients, whether medical or surgical, young or old (Kirkpatrick et al. 2013). Today we understand that IAH and ACS are frequent causes of increased morbidity and mortality (De Waele et al. 2016). More importantly, we now also know that IAH and ACS have correctable causes, can easily be diagnosed and effectively treated, but only if the clinician is aware of these conditions and pursues their recognition (Wise et al. 2015). A monograph has recently been published on this topic, as despite the increasing interest unanswered questions still cloud the understanding of the pathophysiology of IAH and ACS (Malbrain and De Waele 2013). In this article we will try to provide at least some answers.
Understanding Intra-Abdominal Hypertension: What to Worry About?
The abdomen can be considered as
a closed anatomical space with the abdominal contents being primarily fluid in
character, following Pascal’s Law: any change in pressure applied at any
given point is transmitted undiminished throughout the abdomen (Malbrain
2004). This means that intra-abdominal pressure (IAP) can be measured by way
of different direct and indirect routes via the stomach, bladder, uterus or
rectum (Malbrain 2004). The intra-abdominal volume (IAV) will exert a certain
force on the abdominal compartment walls, resulting in a baseline IAP that
will be mainly determined by the abdominal compliance (Cab) (Malbrain 2016).
The relationship between IAV and IAP is curvilinear, with an initial linear
part followed by an exponential increase once a critical volume is reached (Malbrain
et al. 2014a; Malbrain et al. 2014b). IAP is an important physiological
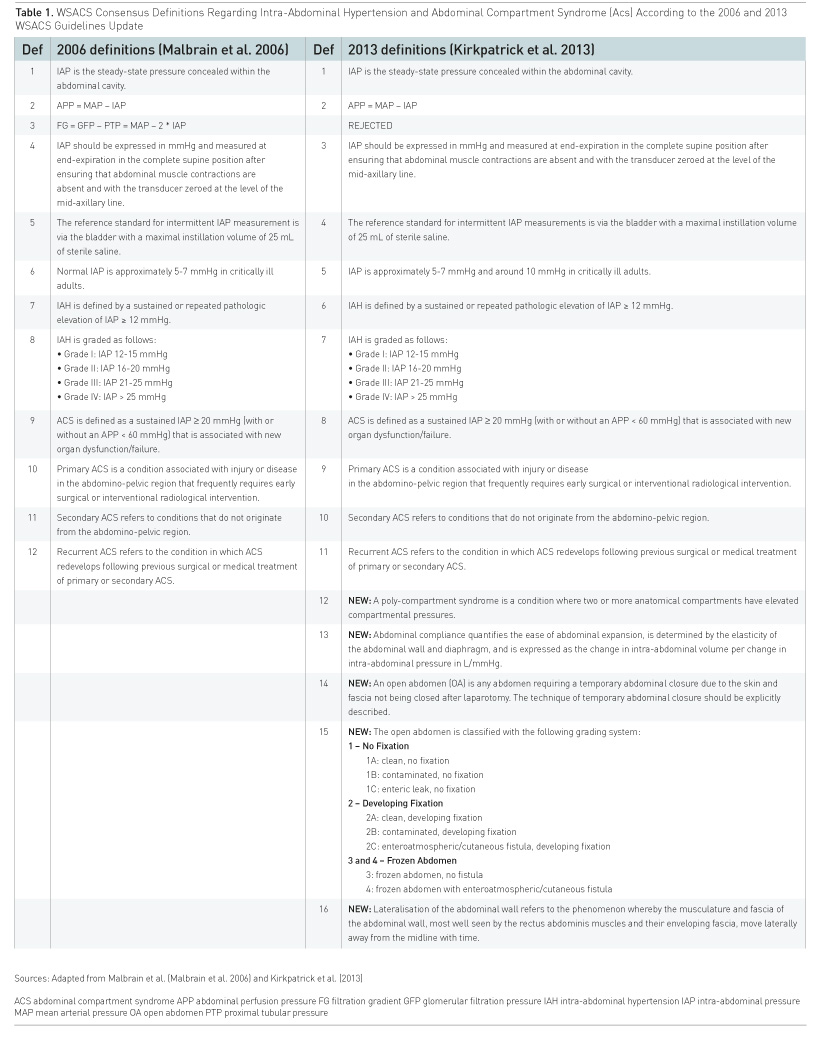
parameter and the recent updated consensus definitions must be used (Table
1) (Kirkpatrick 2013). IAH is defined as a sustained increase in IAP ≥ 12
mmHg and ACS is an IAP > 20 mmHg with new-onset organ failure (Kirkpatrick
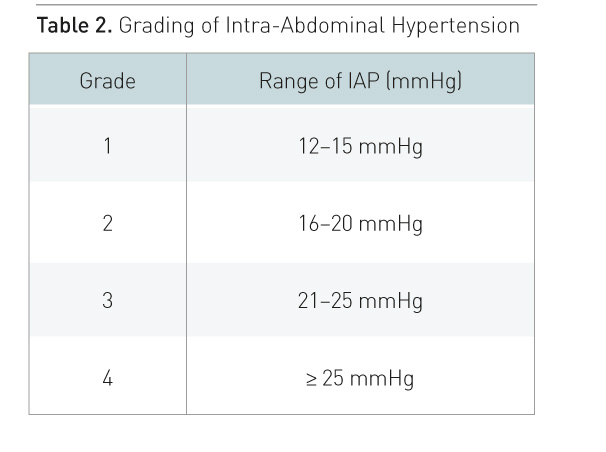
et al. 2013). While IAH is a graded continuum, ACS is an all-or-nothing
phenomenon (Table 2) (Kirkpatrick et al. 2013). IAP should be measured
at end-expiration, with the patient in the supine position and ensuring that
there is no abdominal muscle activity. Intravesicular IAP measurement is
convenient, most widely used and considered the gold standard technique (Kirkpatrick
et al. 2013; Malbrain 2004). Where the mid-axillary line crosses the iliac crest
is the recommended reference level for transvesicular IAP measurement and
marking this level on the patient increases reproducibility of IAP
measurement (Kirkpatrick et al. 2013; De Waele et al. 2008). Instillation
volume (maximal 25 ml) and temperature (above room temperature) may affect
IAP readings, and the head of the bed elevation above 30° increases IAP while
PEEP only minimally affects IAP (Cheatham et al. 2009; Verzilli et al. 2010). Protocols
for IAP measurement should be developed for each intensive care unit (ICU) based
on the locally available tools and equipment, and the ICU physician should
pick the technique that the nurses are going to use. Pitfalls in IAP
measurement are multiple, and thorough knowledge is essential, e.g. absence of
abdominal muscle activity should be checked, particularly in awake patients.
Underlying Predisposing Conditions: When to Worry?
1. Decreased Cab
Clinicians should worry about patients in whom Cab is decreased. The major problem is that Cab is not routinely measured in clinical practice (Malbrain 2014a). However, some indirect measures of Cab are available in mechanically ventilated patients: the ΔIAP (= IAP at end-inspiration minus IAP at endexpiration) and the abdominal pressure variation (APV = mean IAP divided by ΔIAP) are such parameters and they are inversely correlated with Cab, i.e. the higher the ΔIAP or APV, the lower the Cab (Malbrain 2014a). True Cab can only be measured in case of addition or removal of a known abdominal volume (e.g. laparoscopic insufflation, paracenthesis etc.) with simultaneous measurement of the change in IAP. Cab is defined as the ease with which abdominal expansion can occur, and is determined by the elasticity of the abdominal wall and diaphragm (Malbrain 2014a). It should be expressed as the change in IAV per change in IAP (ml/mmHg). Cab helps to understand the pathophysiological mechanisms and possible therapeutic targets (Malbrain et al. 2014a). Increased compliance indicates a loss of elastic recoil of the abdominal wall. Decreased compliance (e.g. in obesity, fluid overload, burn eschars, young age etc.) means that the same change in IAV will result in a greater change in IAP, and this can be a major contributor to secondary IAH.
2. Increased IAV
Clinicians should also worry when IAV is increased: this can be either related to free abdominal fluids or increased intraluminal contents (Kirkpatrick et al. 2013). The relationship between IAV and IAP is expressed by Cab (Malbrain 2016). In patients with IAH, a small increase in IAV can lead to life-threatening aggravation of IAH. Vice versa, in the presence of IAH, a small decrease in IAV can lead to a significant decrease in IAP (Malbrain 2014a). So far, attempts to calculate IAV or to define surrogate markers have failed to prove useful in the clinical setting.
3. Setting of Capillary Leak
The last situation where clinicians should worry is the setting of capillary leak as a result of the inflammatory response and its diverse triggers, including ischaemia–reperfusion injury (Duchesne et al. 2015). Plasma volume expansion to correct hypoperfusion predictably results in extravascular movement of water, electrolytes and proteins. In the context of global increased permeability syndrome this can lead to IAH and sometimes ACS. A variety of strategies are available to the clinician to reduce the volume of fluids used during resuscitation (e.g. by means of active fluid removal or de-resuscitation) (Malbrain et al. 2014c). This may have beneficial effects on IAP and the occurrence of IAH and its related adverse effects (Regli et al. 2015).
Specific
Conditions: When to Worry More?
Normal IAP in mechanically ventilated children is lower than in adults and about 7 mmHg (De Waele et al. 2015). Critical values of IAP that suggest IAH and ACS are also lower in children and an IAP greater than 10 mmHg should be considered as IAH. While IAP above 10 mmHg associated with new organ dysfunction is ACS in children until proven otherwise. IAH and ACS are common in severe acute pancreatitis and one should always suspect IAH in this setting and measure IAP regularly (De Waele et al. 2015). IAP should not be allowed to become greater than 20 mmHg and non-surgical measures should be tried first. However, one should not hesitate to resort to surgical decompression at an early stage if medical management fails (De Keulenaer et al. 2015). IAH will develop in most, if not all, severely burned patients (Wise et al. 2016). One should always suspect IAH and measure the IAP regularly during the initial resuscitation period (Malbrain et al. 2015). The higher the amount of burned surface area and volume of fluid resuscitation the higher the likelihood for developing IAH/ACS. Escharotomy can dramatically reduce IAP in case of circular abdominal burns, while decompressive laparotomy is not a first choice in burn patients. IAH and ACS can occur both in abdominal and extra-abdominal trauma patients. Early recognition in these patients is crucial, and IAP must be measured regularly irrespective of the site of injury. Early bleeding control and avoidance of massive transfusion are key elements in preventing IAH in trauma (Duchesne et al. 2015). Open abdomen treatment should be applied early and liberally in trauma patients at risk for ACS. Medical management strategies to reduce IAP will avoid surgical decompression and complications, and facilitate early closure of the abdomen (De Keulenaer et al. 2015). Baseline IAP is abnormally (chronically) elevated in the morbidly obese patient (Malbrain et al. 2015). Acute elevations in IAP may have similar effects in obese patients, but the threshold before organ dysfunction develops may be higher. Chronic elevations in IAP may, in part, be responsible for the pathogenesis of obesity-related complications (gastro-oesophageal reflux, pulmonary hypertension, pseudotumor cerebri). Pregnancy is another condition with sustained increase in IAP: the higher the IAP, the higher the risk for (pre)eclampsia (Malbrain et al. 2015).
See Also:Long-Term Outcome After Abdominal Compartment Syndrome
Consequences of Intra-Abdominal Hypertension: Why Worry?
The effects of IAH on dfferent organs within and outside the abdomen are well recognised. IAH leads to increased intrathoracic pressure, increased central venous pressure and decreased venous return from the brain (De laet et al. 2007a). As a consequence, increased IAP can lead to increased intracranial pressure in all patients. Prevention of IAH therefore is essential in patients with intracranial hypertension. Cardiovascular dysfunction and failure are common in IAH or ACS (Malbrain et al. 2015b). Accurate assessment of preload, contractility and afterload is therefore essential to restore end-organ perfusion and function. Because pressure-based estimates of intravascular volume are erroneously increased in IAH/ ACS, transmural filling pressures and volumetric preload indicators may better reflect true intravascular preload (Malbrain and Wilmer 2007). IAP also affects chest wall mechanics, and this has clinical relevance during lung protective ventilation (Pelosi et al. 2007). Opening and closing pressures are altered in such a way that a recruitment manoeuvre needs higher pressures and PEEP setting must be adapted to counteract the effects of increased IAP at the level of the diaphragm. IAH is a frequent cause of acute kidney injury (AKI); the relationship between IAP and kidney function seems to be dose-dependent (De Waele et al. 2011; De laet et al. 2007b). Clinically relevant kidney dysfunction may occur at IAP levels as low as 10–12 mmHg, and the best way to prevent IAH-induced AKI is to prevent IAH. Fluid overload should be treated early and aggressively in patients with IAH and AKI, and peritoneal dialysis should be avoided in patients diagnosed with, or at risk for, IAH. Recently the term polycompartment syndrome has been coined alluding to simultaneously increased pressures in different compartments (head, chest, abdomen, extremities etc.) (Malbrain and Wilmer 2007; Malbrain et al. 2014d). Increased compartment pressures are independently associated with morbidity and mortality and clinicians need to be aware of the existence of the polycompartment syndrome and the interactions of increased compartmental pressures between compartments.
Management: How to Stop Worrying?
Based on the underlying conditions that promote IAH and ACS medical management addresses four therapeutic targets:
1. Improving Cab
2. Reducing IAV (either by removing free abdominal or intraluminal fluid)
3. Correcting capillary leak and
4. Correcting fluid balance.
It is beyond the scope of this article to give an extensive overview of the different medical management strategies as these can be found elsewhere (Regli et al. 2015; De Keulenaer et al. 2015). The bottom line is that treatment should always be based equally on the level of IAP, the underlying aetiology, the presence of comorbidities and the degree and magnitude of organ dysfunction.
Conclusions
In 2013 the World Society of the Abdominal Compartment Syndrome (WSACS) published evidence-based guidelines on the definitions, diagnosis and management of IAH and ACS (Kirkpatrick et al. 2013). However, bedside decisions regarding correct management in individual patients with IAH or ACS remain difficult. The clinician should be aware of the polycompartment syndrome and interactions between different compartmental pressures. Cab is one of the most neglected parameters in critically ill patients, although it plays a key role in understanding organ-organ interactions and the deleterious effects of unadapted IAV on IAP and end-organ perfusion
Abbreviations
ACS abdominal compartment syndrome
AKI acute kidney injury
Cab abdominal compliance
IAH intra-abdominal hypertension
IAV intra-abdominal volume
ICU intensive care unit
References:
Cheatham ML, De Waele JJ, De Laet I et al. (2009) The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med, 37(7): 2187-90.
PubMed ↗
De Iaet I, Citerio G, Malbrain ML (2007a) The influence of intraabdominal hypertension on the central nervous system: current insights and clinical recommendations, is it all in the head? Acta Clin Belg Suppl, 62(1): 89-97.
PubMed ↗
De laet I, Malbrain ML, Jadoul JL et al. (2007b) Renal implications of increased intra-abdominal pressure: are the kidneys the canary for abdominal hypertension? Acta Clin Belg Suppl, 62(1): 119-30.
PubMed ↗
De Waele JJ, De Laet I, Malbrain ML (2016a) Understanding abdominal compartment syndrome. Intensive Care Med, 42(6): 1068-70.
PubMed ↗
De Waele JJ, De Laet I, De Keulenaer B et al. (2008) The effect of different reference transducer positions on intra-abdominal pressure measurement: a multicenter analysis. Intensive Care Med, 34(7): 1299-303.
PubMed ↗
De Waele JJ, Ejike JC, Leppaniemi A et al. (2015a) Intra-abdominal hypertension and abdominal compartment syndrome in pancreatitis, paediatrics, and trauma. Anaesthesiol Intensive Ther, 47(3): 219-27.
PubMed ↗
De Waele JJ, De Laet I, Kirkpatrick AW et al. (2011) Intra-abdominal hypertension and abdominal compartment syndrome. Am J Kidney Dis, 57(1): 159-69.
PubMed ↗
Duchesne JC, Kaplan LJ, Balogh ZJ et al. (2015) Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther, 47(2): 143-55.
PubMed ↗
Kirkpatrick AW, Roberts DJ, De Waele J et al. (2013a) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med, 39(7): 1190-206.
PubMed ↗
Malbrain MLNG, De Waele J (2013b) Intra-abdominal hypertension. In: Vuylsteke A, editor. Core critical care series: intra-abdominal hypertension. Cambridge: Cambridge University Press. [Accessed: 24 August 2016] Available from http://www.cambridge.org/catalogue/catalogue.asp?isbn=9780521149396&ss=fro
Malbrain ML (2004) Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med, 30(3): 357-71.
PubMed ↗
Malbrain ML, Peeters Y, Wise R (2016b) The neglected role of abdominal compliance in organ-organ interactions. Crit Care, 20: 67.
PubMed ↗
Malbrain MLNG, De Laet I, De Waele J et al. (2014a) The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 2: Measurement techniques and management recommendations. Anaesthesiol Intensive Ther, 46(5): 406-32.
PubMed ↗
Malbrain MLNG, Roberts DJ, De Laet I et al. (2014b) The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 1: Definitions and pathophysiology. Anaesthesiol Intensive Ther, 46(5): 392-405.
PubMed ↗
Malbrain ML, Marik PE, Witters I et al. (2014c) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther, 46(5): 361-80.
PubMed ↗
Malbrain ML, De Waele JJ, De Keulenaer BL (2015b). What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension. Anaesthesiol Intensive Ther, (4): 388-99.
PubMed ↗
Malbrain ML, De Keulenaer BL, Oda J et al. (2015c) Intra-abdominal hypertension and abdominal compartment syndrome in burns, obesity, pregnancy, and general medicine. Anaesthesiol Intensive Ther, 47(3): 228-40.
PubMed ↗
Malbrain ML, Wilmer A (2007a) The polycompartment syndrome: towards an understanding of the interactions between different compartments! Intensive Care Med, 33(11): 1869-72.
PubMed ↗
Malbrain ML, Roberts DJ, Sugrue M et al. (2014d) The polycompartment syndrome: a concise state-of-the-art review. Anaesthesiol Intensive Ther, 46(5): 433-50.
PubMed ↗
Malbrain ML, Cheatham ML, Kirkpatrick A et al. (2006) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med, 32(11): 1722-32.
PubMed ↗
Pelosi P, Quintel M, Malbrain ML (2007b) Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg Suppl, 62 Suppl 1: 78-88.
PubMed ↗
Regli A, De Keulenaer B, De Laet I et al. (2015a) Fluid therapy and perfusional considerations during resuscitation in critically ill patients with intra-abdominal hypertension. Anaesthesiol Intensive Ther, 47(1): 45-53.
PubMed ↗
Verzilli D, Constantin JM, Sebbane M, Chanques G et al. (2010) Positive end-expiratory pressure affects the value of intra-abdominal pressure in acute lung injury/acute respiratory distress syndrome patients: a pilot study. Crit Care, 14(4): R137.
PubMed ↗
Wise R, Roberts D, Vandervelden S et al. (2015) Awareness and knowledge of intra-abdominal hypertension and abdominal compartment syndrome: results of an international survey. Anaesthesiol Intensive Ther, 47(1): 14-29.
PubMed ↗
Wise R, Jacobs J, Pilate S, Jacobs A et al. (2016) Incidence and prognosis of intra-abdominal hypertension and abdominal compartment syndrome in severely burned patients: pilot study and review of the literature. Anaesthesiol Intensive Ther, 48(2): 95-109.
PubMed ↗




















