ICU Management & Practice, Volume 21 - Issue 6, 2021
The complexity of glucose testing, the limitations of point-of-care blood glucose monitoring systems and the need for accuracy and reliability to ensure optimised patient outcomes.
Critically ill patients are not found just in intensive care units, but throughout the hospital: emergency departments, post-anaesthesia care units, operating rooms, and many other environments now care for the critically ill. These patients require specialised, timely, and individualised care to achieve the best outcomes.
Glycaemic control is a component of care in many, if not most critically ill patients, and the foundation of proper glycaemic control is accurate and timely measurement of blood glucose. Point-of-care testing (POCT) would seem to be a logical solution, but it is not as simple as it appears. Many POC blood glucose monitoring systems (BGMS) simply do not perform adequately in these patients. In fact, only one BGMS has been approved by the U.S. FDA for use in critically ill patients. A major reason for poor performance in some BGMS is from interfering agents. These can be endogenous (anaemia, hypotension), or exogenous such as medications. Drugs which are commonly used in the ICU such as dopamine, acetylcysteine, icodextrin, and ascorbic acid are known to cause falsely elevated glucose readings in most meters.
Anaemia is present in over 70% of ICU patients, and often worsens over time due to phlebotomy (Thavendiranathan et al. 2005). It is also known to cause factitious hyperglycaemia with certain BGMS. While glycaemic control has long been known to play a role in the ICU, it is also being increasingly recognised as important in COVID-19 patients. Diabetes is a comorbidity which increases morbidity and mortality in COVID-19 patients, and well controlled blood glucose in this population is associated with improved outcomes (Zhu et al. 2020). Even in non-diabetic patients with COVID-19, those with uncontrolled hyperglycaemia had a mortality rate of over 40% (Bode et al. 2020).
Clinicians caring for these patients therefore face a difficult situation: send all glucose specimens to the central lab, with its inherent drawbacks (delay in results, preanalytical errors, etc.), or use a BGMS which may give inaccurate results.
Erroneously elevated glucose, regardless of the cause, can lead to overtreatment with insulin, resulting in hypoglycaemia. In fact, at least one death has been reported due to insulin administration in a patient on high dose ascorbic acid who had a falsely high glucose level measured on a BGMS, and there are also numerous case reports of significant and permanent neurologic damage due to inappropriate insulin administration in the setting of other interfering agents (Disque et al. 2013; Jadhav et al. 2013). So, this is not just a theoretical concern. The Italian point-of-care study group recently issued a recommendation not to use BGMS in critical care settings unless the meter is certified for use in critically ill patients (Rampoldi et al. 2021).
One interfering agent that is being utilised more is ascorbic acid (vitamin C). A recent search on clinicaltrials.gov shows over 100 active or recently completed studies using high dose ascorbic acid for COVID-19, sepsis, and burns, among others. One such trial, the LOVIT study (which looks at vitamin C to lessen organ dysfunction) states in its protocol that “Blood glucose can only be measured by one of the following three methods: hospital core laboratory instruments; a point-of-care arterial blood gas machine whose glucose measurement has been validated in the setting of high blood concentration of ascorbic acid; and point-of-care StatStrip glucometers (Nova Biomedical, Waltham, MA, USA), whose measurements have been shown to be accurate in the presence of high blood concentration of ascorbic acid.”(Masse et al. 2020).
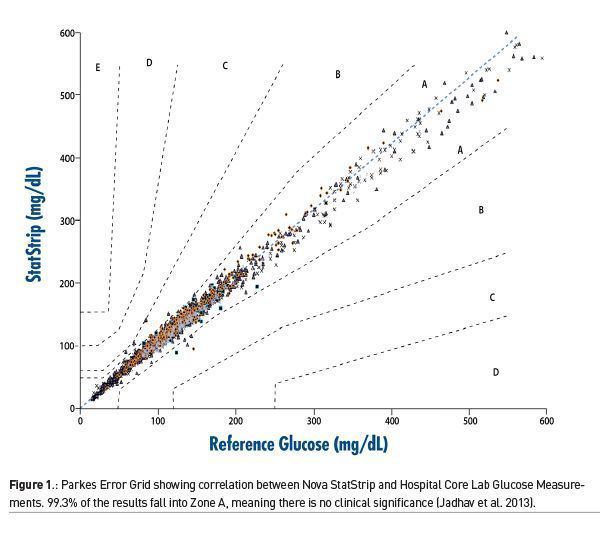
In one large study done in five ICUs in three countries, involving nearly 1700 patients, one BGMS was compared to the hospital central laboratory analyser (DuBois et al. 2017). Using a Parkes Error Grid, 99.3% of results were in Zone A, which means there was no clinical significance in the results between the BGMS and the central lab (Figure 1). The remaining results were in Zone B, representing minimal clinical significance. In addition, the performance of the BGMS met the most current standard for glucose measurement as defined by CLSI POCT12-A3 with 95.4% of results <100mg/dL and 96.5% of the results >100 mg/dL falling within 12 mg/dL of the core lab. No significant interferences were identified in any patient.
Given the complexity of glucose testing it is imperative for clinicians and laboratorians to be aware of the limitations of any BGMS that is being used in settings where critically ill patients are cared for. In a high-risk environment, where accuracy and reliability can directly impact patient outcomes, not all BGMS devices are created equally.
Key Points
- Glycaemic control is a component of care in many, if not most critically ill patients.
- Point-of-care testing would seem to be a logical solution, but many POC blood glucose monitoring systems do not perform adequately in these patients.
- Erroneously elevated glucose, regardless of the cause, can lead to overtreatment with insulin, resulting in hypoglycaemia.
- Given the complexity of glucose testing it is imperative for clinicians and laboratorians to be aware of the limitations of any BGMS that is being used in settings where critically ill patients are cared for.
References:
Bode B et al. (2020) Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol, 14(4):813-821.
Disque A, Dhillon A, Gritsch A (2013) Icodextrin causing glucose meter error and severe hypoglycemia after deceased donor renal transplant in a patient receiving continuous ambulatory peritoneal dialysis. AA Case Rep, 1(6):89-91.
DuBois JA et al. (2017) Bedside Glucose Monitoring-Is it Safe? A New, Regulatory-Compliant Risk Assessment Evaluation Protocol in Critically Ill Patient Care Settings. Crit Care Med, 45(4):567-574.
Jadhav PP, Jadhav MP (2013) Fallaciously elevated glucose level by handheld glucometer in a patient with chronic kidney disease and hypoglycemic encephalopathy. International Journal of Case Reports and Images, 4(9):485-488.
Masse M.-H et al. (2020) Lessening Organ dysfunction with VITamin C (LOVIT): protocol for a randomized controlled trial. Trials, 21(1):42.
Rampoldi E et al. (2021) Principi per l’implementazione e la gestione del point-of-care-testing (POCT): indicazioni essenziali. biochimica clinica, 45(3):312-326.
Thavendiranathan P et al. (2005) Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med, 20(6): 520-4.
Zhu L et al. (2020) Association of Blood Glucose Control and Outcomes in Patients with COVID- 19 and Pre-existing Type 2 Diabetes. Cell Metab, 31(6):1068-1077.e3.
while glycaemic




















