Defining the Role of the Intensive Care Unit
This review article aims to alter the preconceived mindset that surrounds the intensive care unit (ICU) and the patient with an acute ischaemic stroke (AIS). A contemporary body of evidence is emerging that shows that specific interventions can improve outcomes, and this article highlights key evidence-based strategies in AIS management. More importantly, it focuses on the broader management facets such as the standards of AIS care, stroke care pathways and indications for ICU admission. When compared with other serious ICU diagnoses such as severe sepsis and long term ventilation, the outcome of AIS patients on ICU compares well. This comparison should shake the historical reluctance that surrounds admission of stroke patients to ICU.
Stroke is the second largest contributor
to mortality worldwide. Its devastating
consequences are also a major contributor
to morbidity, especially in high-income countries,
where it is a leading cause of years of life
lived with disability (Lopez et al. 2006). Historically,
acute ischaemic stroke (AIS) was largely
seen as an irreversible condition with reports
previously suggesting no significant reduction
in morbidity and mortality following intensive
care unit (ICU) admission (Drake et al. 1973;
Kennedy et al. 1970). Modern-day paradigms
have changed however with an established,
contemporary body of evidence showing that
specific interventions can improve outcomes.
As a consequence, thresholds for admitting AIS
patients to ICU to support these interventions,
and their potential complications, are falling. A
recent analysis of 31,301 AIS patients admitted
to hospital in the eastern half of the United
States showed that 26% required ICU admission
at some stage (Golestanian et al. 2009).
Despite this, there seems to be a lack of widely
accepted indications for admission to the ICU
and evidence for management thereafter.
Standards of Care and Stroke Care
Pathways
Management of acute stroke is complex. Interventions
such as prompt diagnosis, consideration
for thrombolysis, correction of deranged
physiology and secondary prevention must be
coordinated in a timely fashion. Care pathways
assist healthcare professionals in making clinical
decisions according to the best available
evidence, thereby improving patient care and
reducing variation in clinical practice. Their
place in the acute management of AIS has therefore
become commonplace. Some of the typical
components of an AIS care pathway include
(Intercollegiate Stroke Working Party 2012;
National Collaborating Centre for Chronic
Conditions (UK) 2008):
• If indicated, “immediate” brain imaging
(ideally the next slot and definitely
within one hour);
• If indicated, thrombolysis with alteplase
within 4.5 hours;
• Direct admission to a specialist acute
stroke unit;
• Antiplatelet treatment to start as soon as
possible, and certainly within 24 hours.
The evaluation of care pathways in acute
stroke is complicated however, given regional
variability in content and delivery. A recent
Cochrane review (Stroke Unit Trialists’ Collaboration
2013) looked at the use of care pathways
in acute stroke management and rehabilitation.
Of the 15 studies, which included more than
4,000 patients, only three were randomised.
The primary outcome measures of death and
dependency at discharge showed no clear
benefit with the use of care pathways. Mindful
that non-randomised studies are susceptible to
biases and clinical studies of care pathways are
open to confounding, this Cochrane review still
suggests that the benefits of AIS care pathways
are not proven.
If care pathways are to succeed, they are only
likely to improve standards of care if implemented
in specialised stroke units. Such units
confer a clear morbidity and mortality benefit
over general medical care and should form the
standard of care. This benefit does not seem to
be limited by age, sex, type or severity of stroke
(Stroke Unit Trialists’ Collaboration 2013).
Where such units exist, cohesive multidisciplinary
input ranging from paramedics
and emergency physicians through to stroke
specialists, interventional radiologists and
surgeons are key to drive standards set in the
care pathway. Intensivists are playing increasing roles within these teams, ensuring that provision
of optimal organ support and specialised
nursing care continue in parallel to other
ongoing stroke management. The delay in
transfer of critically ill stroke patients from the
emergency department to the neurointensive
care has been shown to be an independent
indicator of poor outcome at hospital discharge
(Rincon et al. 2010).
Appropriate training tools can also improve
standards of acute stroke care. Simulation-based
models have been used to improve utilisation
of thrombolysis by helping to identify barriers
along care pathways (for example picking up
delays in ambulance transfers) and provide
solutions for these (for example the scoop-andrun
protocol) (Lahr et al. 2013). NavarreteNavarro
et al. (2012) also showed that the
introduction of a training model (e-learning
course, lectures and workshops) focusing on
therapeutic and organisational aspects of AIS
management led to improved knowledge of
emergency and critical care physicians and
formed part of the regional strategy on stroke
management, leading to increased uptake of
thrombolysis in the region.
Indications for ICU Admission
The decision to admit an AIS patient to ICU is
often to improve/support blood flow to the
ischaemic penumbra. This is achieved through
reperfusion therapies, optimisation of neuroprotective
strategies and the support of other
organs during neurological recovery. Other
indications include prevention, early detection
and treatment of complications and the
need for close monitoring. Before making a
final decision on appropriateness for admission,
prior co-morbidity, cognitive and functional
status and personal wishes should also be taken
into consideration.
Accurate neurological prognostication is
central to the decision to admit, but is notoriously
difficult. Detailed history taking, thorough
examination and appropriate imaging are
key, but may not predict for all. Stroke mimics
such as psychogenic disorders, hypoglycaemia,
seizures, complicated migraine, encephalopathy,
central nervous system mass lesions and
drug toxicity need to be excluded. Neurological
prognostication is near to impossible during
the acute phase and is more accurately determined
through repeated assessments temporally,
involving regular discussion between ICU
and stroke physicians (Kirkman et al. 2014).Therefore the American Stroke Association
recommends aggressive treatment and postponement
of “Do Not Attempt Cardiopulmonary
Resuscitation” (DNACPR) orders for at
least the first 24 hours (Jauch et al. 2013).
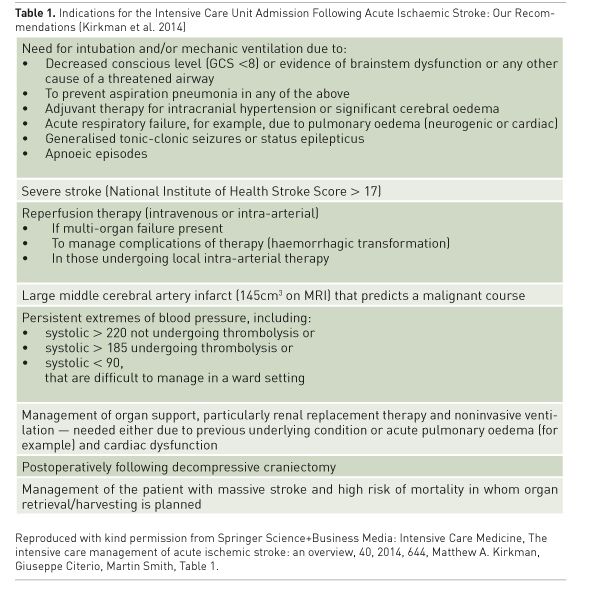
Kirkman et al. recently reviewed current AIS
guidelines and produced recommendations
on indications for ICU admission as shown in
Table 1 (Kirkman et al. 2014). While patients
with AIS, decreased level of consciousness and
a National Institutes of Health Stroke Score
(NIHSS) > 17 on admission are thought to
have a poor prognosis, exceptions exist, such
as the response seen by cerebellar infarcts to
sub-occipital craniectomy (Kirkman et al.
2014; Wijdicks et al. 2014). The relationship
between stroke severity and outcome should
be observed with caution as patients with
more severe deficits will inherently have the
most to gain from treatment, especially when
compared with mild strokes where death or
severe disability have been used as the primary
outcomes of research (Stroke Unit Trialists’
Collaboration 2013).
The need for respiratory support is one of
the more common causes for ICU admission
in patients with AIS. While the literature is not
clear, a few small trials indicate that patients
who are intubated and ventilated for neurological
deterioration (coma) and respiratory
deterioration do not do as well as those who
are intubated and ventilated for potentially
reversible causes such as seizure management
or prevention of aspiration pneumonia (Burtin
et al. 1994; Leker and Ben-Hur 2000; Meyfroidt
et al. 2014; Steiner et al. 1997; Wijdicks and
Scott 1997).
Therapeutic Strategies in
AIS Management
The initial supportive management of AIS is
not complex and should not intimidate the
ICU practitioner. Even in ICU, the simple task
of ensuring that all the small facets of stroke
care are done well can have the greatest benefit
to our patients by avoiding secondary brain
damage. Supplemental oxygen therapy targeted
to oxygen saturation (to avoid both hypoxia
and hyperoxia), avoidance of fever and glucose
control are paramount. Both high and low
blood pressure during an AIS are independent poor prognostic factors for outcome (LeonardiBee
et al. 2002). It is necessary to acutely lower
blood pressure to less than 185/110mmHg
to enable thrombolysis. If treatment does
not include thrombolysis, only blood pressures
greater than 220/120mmHg should be
gently reduced by no more than 15% per 24
hours, except if co-morbidities such as severe
cardiac failure, aortic dissection or hypertensive
encephalopathy occur (European Stroke
Organisation (ESO) Executive Committee and
ESO Writing Committee 2008). AIS patients
with very high or labile blood pressures, or
patients who are being mechanically ventilated
should have continuous invasive arterial blood
pressure monitoring. Intravenous labetalol is
most commonly recommended, but intravenous
nicardopine or glycerine trinitrate may
all be used to cautiously lower blood pressure.
A recent trial has shown that more aggressive
systolic blood pressure lowering to around
140mmHg is safe but confers no benefit (He
et al. 2014).
Early aspirin therapy (within 48 hours) seems
to confer a small benefit with fewer deaths and
less stroke recurrence without an increase in
haemorrhagic complications (CAST (Chinese
Acute Stroke Trial) Collaborative Group 1997).
Aspirin therapy should not however be used
within 24 hours of thrombolysis (Jauch et al.
2013). Although immediate treatment with
subcutaneous heparin is associated with less
recurrent ischaemic strokes, it is associated
with more haemorrhagic strokes and therefore
AIS patients are not therapeutically anticoagulated
for at least the first two weeks after
their stroke and preferably after liaison with a
haematologist.
AIS patients are at high risk of deep vein
thrombosis (DVT) and pulmonary embolus
(Jauch et al. 2013). This risk may be reduced
through hydration and early mobilisation.
However, the use of prophylactic subcutaneous
low molecular weight heparin should
be avoided for at least 24 hours after thrombolysis,
and is commonly withheld for 2 weeks
following an AIS for fear of potentiating a
haemorrhagic transformation. The exact timing
of initiating low molecular weight heparins
is unclear and further research in this area is
underway. The use of intermittent pneumatic
compression is an effective method of reducing
DVTs and shows a trend to reduced mortality,
while graduated compression stockings do
not reduce thromboembolic events and may
cause skin tears and are therefore best avoided (CLOTS (Clots in Legs Or sTockings after
Stroke) Trials Collaboration et al. 2013).
Two interventions that alter the natural
course of AIS, which are both backed by level
1 evidence, are worthy of discussion and
should be actively facilitated and supported
where necessary with ICU admission. First,
thrombolysis with intravenous recombinant
tissue plasminogen activator (rTPA), after
clinical and radiographic diagnosis of AIS. This
should be given within a four-and-a-half-hour
window and be instituted as soon as possible.
Endovascular alternatives (e.g. clot retrieval,
stenting) are gaining in popularity. New
evidence suggests that some patients with AIS
and moderate to severe neurological impairment,
with very proximal occlusions, benefit
from clot retrieval and stenting, demonstrated
by improved outcomes beyond that possible
by thrombolysis alone (Prabhakaran et al.
2015). Time to reperfusion seems to be the
most crucial factor, irrespective of the method
used. However, this field is moving fast and
indications and preferences are likely to change.
Secondly, patients with large infarctions who
are at risk of malignant cerebral oedema should
be monitored closely, and early referral to a
unit with neurosurgical capabilities should be
discussed as soon as possible. Patients under
the age of 60 with malignant MCA infarcts
and cerebral oedema have improved outcome
if decompressive craniectomy is achieved
within 48 hours (Vahedi et al. 2007). The
recent Decompressive Surgery for the Treatment
of Malignant Infarction of the Middle
Cerebral Artery (DESTINY) II trial has illustrated
increased survival, but some survivors
are often left with substantial disability (Jüttler
et al. 2014) and surgery should be considered
with caution especially in advancing age.
Comparing Outcomes - Is it Worth it?
One of the main reasons for refusing an AIS
patient admission to the ICU is the perceived
futility of the admission. Navarrete-Navarro
et al. conducted a multicentre, prospective
observational study in 28 Spanish hospitals that
recorded the mortality and disability of 132
ICU-admitted severe stroke patients. Patients
with AIS had the highest inpatient survival rate
of 78%, but this decreased to 34% after one
year and only 25% of patients had minimal or
no disability at one year (Navarrete-Navarro
et al. 2003). This data is similar to critical care
outcomes at one of the largest acute stroke
units in London, which followed up 144 patients over two years and found an ICU
survival rate of 62% and a one-year survival
rate of 30% in patients with AIS. Importantly
over 60% of these survivors had a favourable
neurological outcome (unpublished data).
These outcomes are not markedly different
from other groups of critically ill patients. A
recent prospective analysis of severe sepsis survivors
showed comparable mortality outcomes as
well as cognitive and functional disability rates.
This large nationally representative cohort of
more than 1,194 patients over the age of 50
revealed a 90-day mortality after severe sepsis
of 41%. The odds of acquiring a moderate to
severe cognitive impairment were 3.3 times
more likely following sepsis when compared
with a general hospital admission. Furthermore,
there was a mean increase of 1.5 new functional
limitations following sepsis (Iwashyna et
al. 2010). Similarly, studies have shown that only
9% of long-term (median of 27 days) ventilated
patients reach independent functioning at one
year (Unroe et al. 2010).
Stroke should therefore be viewed in the same
light as other severe conditions requiring ICU
admission, including severe sepsis and longterm
ventilation. Rapidly evolving strategies
that aggressively alter the natural course of
stroke hope to further improve stroke outcomes
in the near future.
Conclusion
Stroke is a major contributor to mortality and
morbidity worldwide. Modern-day attitudes
and paradigms are shifting as a rapidly growing
body of evidence emerges. As a result, there is
generally less reluctance to admit AIS patients
to ICU, and outcomes compare well with other
serious ICU conditions. Effective management
of the critically ill stroke patient requires proactive,
rapid and coordinated decision-making
by a multidisciplinary team, including stroke
physicians and nurses, intensivists and radiologists.
This teamwork does not always come
naturally; education, regular training, systems
and support need to be put in place to ensure
that the correct resources are rapidly bought to
bear on one of the most time-critical medical
emergencies. It is hoped that future trials will
identify further medical interventions and
better ways to structure stroke units to facilitate
better outcomes.
Burtin P, Bollaert PE, Feldmann
L et al. (1994) Prognosis of stroke patients undergoing mechanical ventilation.
Intensive Care Med, 20(1): 32–6.
CAST
(1997) CAST: randomised placebo-controlled trial of early aspirin use in 20,000
patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial)
Collaborative Group. Lancet, 349(9066): 1641–9.
CLOTS
(Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M,
Sandercock P et al. (2013) Effectiveness of intermittent pneumatic compression
in reduction of risk of deep vein thrombosis in patients who have had a stroke
(CLOTS 3): a multicentre randomised controlled trial. Lancet, 382(9891):
516–24.
Drake
WE, Hamilton MJ, Carlsson M et al. (1973) Acute stroke management and patient
outcome: the value of Neurovascular Care Units (NCU). Stroke J Cereb Circ, 4(6):
933–45.
European
Stroke Organisation (ESO) Executive Committee, ESO Writing Committee (2008)
Guidelines for management of ischaemic stroke and transient ischaemic attack
2008. Cerebrovasc Dis, 25(5): 457–507.
Golestanian
E, Liou JI, Smith MA (2009) Long-term survival in older critically ill patients
with acute ischemic stroke*: Crit Care Med, 37(12): 3107–13.
He
J, Zhang Y, Xu T, Zhao Q et al. (2014) Effects of immediate blood pressure
reduction on death and major disability in patients with acute ischemic stroke:
the CATIS randomized clinical trial. JAMA, 311(5): 479–89.
Intercollegiate
Stroke Working Party (2012) National clinical guideline for stroke, 4th
Edition, London: Royal College of Physicians. [Accessed 9 September 2015]
Available from rcplondon.ac.uk/guidelines-policy/stroke-guidelines
Iwashyna
TJ, Ely EW, Smith DM et al. (2010) Long-term cognitive impairment and functional
disability among survivors of severe sepsis. JAMA, 304(16): 1787–94.
Jauch
EC, Saver JL, Adams HP et al. (2013) Guidelines for the early management of
patients with acute ischemic stroke: a guideline for healthcare professionals
from the American Heart Association/American Stroke Association. Stroke, 44(3):
870–947.
Jüttler
E, Unterberg A, Woitzik J, et al. (2014) Hemicraniectomy in older patients with
extensive middle-cerebral-artery stroke. N Engl J Med, 370(12): 1091–1100.
Kennedy
FB, Pozen TJ, Gabelman EH et al. (1970) Stroke intensive care--an appraisal. Am
Heart J, 80(2): 188–96.
Kirkman
MA, Citerio G, Smith M (2014) The intensive care management of acute ischemic
stroke: an overview. Intensive Care Med, 40(5): 640–53.
Lahr
MMH, van der Zee DJ, Luijckx GJ et al. (2013) A simulation-based approach for
improving utilization of thrombolysis in acute brain infarction. Med Care, 51(12):
1101–5.
Leker
RR, Ben-Hur T (2000) Prognostic factors in artificially ventilated stroke
patients. J Neurol Sci, 176(2): 83–7.
Leonardi-Bee
J, Bath PMW, Phillips SJ et al. (2002) Blood pressure and clinical outcomes in
the international stroke trial. Stroke, 33(5): 1315–20.
Lopez
AD, Mathers CD, Ezzati M et al. (2006) Global
and regional burden of disease and risk factors, 2001: systematic
analysis of population health data. Lancet, 367(9524): 1747–57.
Meyfroidt
G, Bollaert PE, Marik PE (2014) Acute ischemic stroke in the ICU: to admit or
not to admit? Intensive Care Med, 40(5): 749–51.
National
Collaborating Centre for Chronic Conditions (UK) (2008) Stroke: National
Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and
Transient Ischaemic Attack (TIA), National Institute for Health and Clinical
Excellence: Guidance. London: Royal College of Physicians.
Navarrete-Navarro
P, Murillo-Cabeza F, Bono-de-Seras R et al. (2012) Development of an acute
ischemic stroke management course for hospital physicians in emergency
departments and intensive care units. Eur J Emerg. Med, 19(2): 108–11.
Navarrete-Navarro
P, Rivera-Fernández R., López-Mutuberría MT, Galindo
I et al. (2003) Outcome prediction in
terms of functional disability and mortality at 1 year among ICU-admitted
severe stroke patients: a prospective epidemiological study in the south of the
European Union (Evascan Project, Andalusia, Spain). Intensive Care Med, 29(8): 1237–44.
Prabhakaran
S, Ruff I, Bernstein RA (2015) Acute stroke intervention: a systematic review.
JAMA, 313(14): 1451-62.
Rincon
F, Mayer SA, Rivolta J et al. (2010) Impact of delayed transfer of critically
ill stroke patients from the Emergency Department to the Neuro-ICU. Neurocrit
Care, 13(1): 75–81.
Steiner
T, Mendoza G, De Georgia M et al. (1997) Prognosis of stroke patients requiring
mechanical ventilation in a neurological critical care unit. Stroke J Cereb
Circ, 28(4): 711–5.
Stroke
Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for
stroke. In: The Cochrane Collaboration (Ed.), Cochrane Database of Systematic
Reviews (UK) [Internet]. Chichester: John Wiley & Sons Ltd 2013- [cited
2015 September 9] Available from http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000197.pub3/full
Unroe
M, Kahn JM, Carson SS et al. (2010) One-year trajectories of care and resource
utilization for recipients of prolonged mechanical ventilation: a cohort study.
Ann Intern Med, 153(3): 167–75.
Vahedi
K, Hofmeijer J, Juettler E et al. (2007) Early decompressive surgery in
malignant infarction of the middle cerebral artery: a pooled analysis of three
randomised controlled trials. Lancet Neurol, 6(3): 215–22.
Wijdicks
EF, Sheth KN, Carter BS et al. (2014) Recommendations for the management of
cerebral and cerebellar infarction with swelling: a statement for healthcare
professionals from the American Heart Association/American Stroke Association.
Stroke, 45(4): 1222–38.
Wijdicks
EF, Scott JP (1997) Causes and outcome of mechanical ventilation in patients
with hemispheric ischemic stroke. Mayo Clin Proc, 72(3): 210–13.






















