ICU Management & Practice, Volume 18 - Issue 1, 2018
There is still no widespread agreement around optimal
targets for glucose control in the ICU: some clinicians maintain that glucose
control is unnecessary and harmful, while others claim that blood glucose
control is essential to improve prognosis.1-3
Those who favour liberal glycaemic control assert that hyperglycaemia is simply a beneficial adaptation in critically ill patients to provide fuel for vital organ systems. This view is supported by results from the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation) study which concluded that tight glycaemic control (TGC) led to moderate and severe hypoglycaemia and an increased risk of death.4
The other view is that hyperglycaemia, in the context
of early nutrition, is maladaptive and harmful. Glucose overload in cells that
do not need insulin for glucose uptake may cause mitochondrial damage and,
while hypoglycaemia is a risk, it can be prevented.
To explore the
relationship between blood glucose levels and prognosis, we carried out two
studies, using an identical design, on patients entering either the adult
surgical ICU (S-ICU) or adult medical ICU (M-ICU).5,6In
both studies, patients were randomly assigned to receive intensive insulin
therapy (IIT: target maintenance level 80 to 100mg/dL) or conventional
treatment (insulin only when blood glucose was between 180 and 215mg/dL and
stopped again when falling below 180mg/dL). IV glucose was given on admission,
followed on day 2 by early standardised parenteral (PN) combined with enteral
nutrition (EN).
Pooling the results for the 2748 patients, IIT was
associated with a clear reduction in hospital mortality of 4% (p=0.02) in the
total population and 8% (p=0.006) among those patients who were in the ICU for
at least 3 days.7
It was unclear whether maintenance of normoglycaemia or
administration of insulin contributed to the clinical benefits but a
subsequent animal study suggested that most benefits were due not to the
administration of insulin but to the avoidance of hyperglycaemia.8Glycaemia-independent effects of insulin were evident only when
normoglycaemia was maintained.
Further studies found that hyperglycaemia brought about cellular glucose overload in the kidney, which was associated with mitochondrial dysfunction and renal injury. Histological examination showed clear flattening of the tubules with loss of tubular epithelium and intraluminal debris or calcification in kidneys from the hyperglycaemia group.9It was also found that hyperglycaemia induced cellular glucose overload in the liver and myocardium, causing mitochondrial dysfunction.10
In 7/9 human patients who had been treated
conventionally there were enlarged mitochondria in liver samples with increased
abnormal and irregular cristae. Only 1/11 patients given intensive insulin
therapy displayed these abnormalities (p=0.005).11The
authors noted that the lack of effect on skeletal-muscle mitochondria suggested
a direct effect of glucose toxicity rather than of insulinaemia.
Numerous
mechanistic studies have confirmed that cells which are not dependent on
insulin for glucose uptake can experience mitochondrial damage from
hyperglycaemia in the context of critical illness.
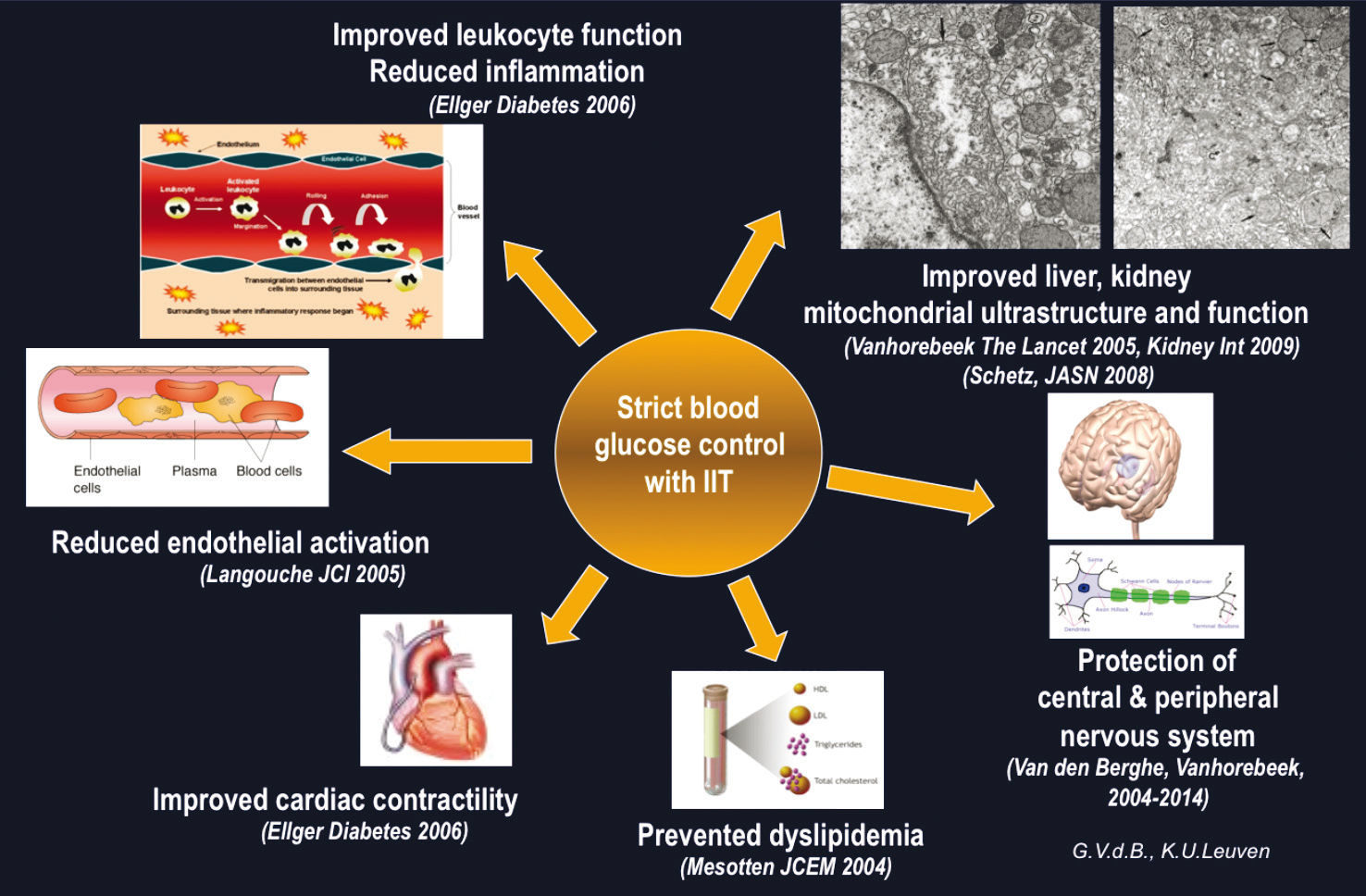
A post hoc analysis of the results from the S-ICU and
M-ICU studies found a marked survival benefit of intermediate vs limited
control, and a slight further improvement with tight control, while only tight
control produced very marked benefits in terms of new kidney injury.5-7 With polyneuropathy, benefi t was only seen with the
tightest control.
In children, it is critical to target relevant age-adjusted values; targeting adult fasting levels of glucose may be harmful or, at best, ineffective. Seven hundred critically ill infants and children who were admitted to the paediatric ICU (PICU) were randomly assigned to target blood glucose levels (2.8 to 4.4mmol/L for infants, 3.9 to 5.6mmol/L for children) with insulin infusion throughout PICU stay, or to a second group where insulin infusion was used only to prevent blood glucose exceeding 11.9mmol/L.12BG was brought down to fasting levels in the intensive group and benefi ts of tight control were seen in multiple areas including shorter PICU stay (5.51 vs 6.15 days, p=0.017), lower C-reactive protein (9.75mg/L vs 8.97mg/L, p=0.007) and fewer infections (29.2% vs 36.8%, p=0.034). Hypoglycaemic episodes must be carefully managed to avoid rebound to high glucose levels. A comparison of children who had experienced hypoglycaemic episodes with those who had not, revealed no adverse effects on IQ, visual-motor integration or executive functions.
This contrasts with the findings of the NICE-SUGAR
study which reported an increased risk of death in ICU patients who had
experienced a hypoglycaemic episode.4This was attributed
not to any effect on organ function, rather to cardiovascular failure.
Several important
differences between the studies need to be highlighted. First, different target
levels were used, with the two study arms in the NICE- SUGAR study being much
closer together. Compliance levels also differed: in the Leuven studies, 70% of
patients in the intervention group achieved target levels compared to less than
50% in the NICE-SUGAR study.
Many of the glucometers allowed in the NICE-SUGAR study have
since been found to be unsuitable for their purpose.13,14Inaccuracies in measuring glucose can result in poor insulin
titration and lead to large BG fluctuations and undiagnosed hypoglycaemia.
Further errors could have been introduced through use of a too simple “if-then”
algorithm that could be adapted or even set aside, unlike the computerised
algorithm that has been developed at Leuven.15
Finally, in the NICE-SUGAR study, feeding was almost entirely
via the enteral route whereas at Leuven, inadequate enteral feeding was supplemented
with PN. It is possible that the NICE-SUGAR feeding protocol induced global
substrate deficit through insulin-induced suppression of metabolism. On the
other hand, PN in the Leuven studies may have increased the severity of
stress-induced hyperglycaemia, with insulin infusion being required to counter
the effect.
To investigate this, two RCTs were conducted to compare early
vs late PN in critically ill adults (EPaNIC) and children (PEPaNIC).16,17Both studies produced similar results, more pronounced in
children, with patients experiencing more infections and a lower likelihood for
live discharge with early PN. In a secondary analysis it was found that delayed
recovery with early feeding was explained by the amount of proteins or amino
acids consumed, rather than glucose.18The likely
mechanism is that amino acids suppress autophagy, a process which eliminates
mitochondria that are damaged by hyperglycaemia.
Given
these insights, our proposal is to re-do our original randomised controlled
studies but under different conditions. In particular we will target fasting
blood levels against hyperglycaemia up to 215mg/dL, and will not include early
PN. This study, the “TGC-fast” study has received funding and is now in the
process of being set up to recruit almost 10,000 patients.
Satellite Symposium Proceeding from ESICM 2017
30th Annual Congress – Vienna, 25th September 2017
References:
2. Gunst J. Intensive Care Med 2016;42:1478-1.
3. Preiser JC. Intensive Care Med 2016;42:1482-4.
4. Finfer SR. N Engl J Med 2012;367:1108-18.
5. Van den Berghe G. N Engl J Med 2001;345:1359-67.
6. van den Berghe G. N Engl J Med 2006;354:449-61.
7. Van den Berghe G. Diabetes 2006;55:3151-9.
8. Ellger B. Diabetes 2006;55:1096-105.
9. Vanhorebeek I. Kidney Int 2009;65:512-20.
10. Vanhorebeek I. Crit Care Med 2009;37:1355-64.
11. Vanhorebeek I. Lancet 2005;365:53-9.
12. Vlasselaers D. Lancet 2009:doi:10.1016/S0140-6736(09)60044-1.
13. Cembrowski GS. Clin Chem 2010;June:doi: 10.1373/clinchem.2010.146217.
14. Scott MG. Clin Chem 2009;55:18-20.
15. van Herpe T. Diab Care 2013;36:188-94.
16. Casaer MP. NEJM 2011;365:506-17.
17. Fivez T. N Engl J Med 2016;374.
18. Casaer MP. Am J Respir Crit Care Med 2012;November 29:Epub ahead of print.



















