ICU Management & Practice, ICU Volume 14 - Issue 2 - Summer 2014
Authors

Tim M. Cook, BA (Hons), MBBS (Hons), FRCA, FFICM
Consultant in Anaesthesia and Intensive Care Medicine

Jeremy P. Astin, MBChB (Hons), BSc (Hons), MRCP, FRCA
Specialist Registrar in Anaesthesia

Fiona E. Kelly, BA (Hons), MBBS, MRCP, FRCA, DICM, FFICM
Consultant in Anaesthesia and Intensive Care Medicine
Royal United Hospital Combe Park, Bath, UK
[email protected]
By three methods we may learn wisdom: the first by reflection, which is the noblest; the second by imitation, which is the easiest; and third by experience which is the bitterest - Confucius.
The 4th National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society; major complications of airway management in the UK (NAP4) reported that the intensive care unit (ICU), rather than being a place of safety for airway management, could instead be considered a place of danger (Cook et al. 2011a). Airway-related complications were more likely to occur on ICU than in theatre, and more likely to result in patient harm: the rate of airway-related complications on ICU was more than 50 times that during anaesthesia, and 50% of patients reported to NAP4 from ICU died, compared to 14% of those reported from theatres (Cook et al. 2011a; Whitaker 2011). Whereas most airway complications during anaesthesia arose at intubation (Cook et al. 2011a), the majority of life-threatening airway events on ICU involved accidental airway dislodgement, especially of tracheostomies. Difficulties associated with rapid sequence intubation and failures of airway rescue techniques were also seen.
Human factors were documented in 40% of reports to NAP4 (Cook et al. 2011a), and further analysis suggests the true figure is 100% (Flin et al. 2013). NAP4 highlighted organisational (latent) failings (e.g. lack of equipment, policies, training and staffing) and individual (active) errors (e.g. lack of knowledge or structured approach, omissions, loss of situation awareness, judgement errors), both of which were more prominent in cases occurring on ICU than during anaesthesia.
This article focuses on recent developments in four areas of ICU airway care identified in NAP4:
• Tracheostomy;
• Human factors;
• Capnography;
• Videolaryngoscopy.
Tracheostomy
Prior to NAP4, much of the focus of research around tracheostomy and ICU related to novel tracheostomy techniques, reducing procedural complications and determining optimum timing. In 2013 the TracMan study, which randomised patients to tracheostomy at <4 and >10 days, reported no differences in early or late allcause mortality, length of ICU stay or procedural complications (6.3%) (Young et al. 2013). A recent metaanalysis also reported no effect on ventilator-associated pneumonia, ICU stay or mortality (Huang et al. 2014).
Remarkably, there is little data on the prevalence of tracheostomy in modern ICU care. A Swiss survey, in 2000, reported tracheostomy in 10% of patients ventilated >24 hours and 1.3% of all ICU patients (Fischler et al. 2000), while an English estimate in 2012 suggested tracheostomy formation in 9% of all adult ICU admissions (McGrath et al. 2012b). It is likely that practices have changed dramatically since then, and also vary across and within different European countries.
NAP4 identified tracheostomy-related complications, mainly tracheostomy displacement, particularly in obese patients, as important factors in major airway complications on ICU (Harper et al. 2011a). This built on previous work by Thomas and McGrath. In 2009 they reviewed >1000 ICU airway safety incidents submitted to the UK critical incident reporting system: 1 in 10 led to longer term harm, with the vast majority occurring after the airway had been established (Thomas and McGrath 2009). In their series, infants, equipment problems and tracheostomy were over-represented. Harm due to partial airway displacement and failure to use capnography were notable. They also examined ≈500 tracheostomy-related patient safety incidents on wards (McGrath and Thomas 2010). Displacement and blockage were prominent and patient harm was common, with long term harm in 19% cases. Of note, reporting systems usually underestimate safety incidents causing patient harm by up to 20-fold (Sari et al. 2007).
The variable design of tracheostomy tubes, particularly those suitable for obese patients, was highlighted by NAP4 (Harper et al. 2011b). Encouragingly equipment manufacturers are actively seeking to engage in redesign of tracheostomy tubes (personal communication
medical manufacturer).
Concerns about management of tracheostomies extend beyond ICU, and several important quality improvement initiatives are in development. In the UK, the National Tracheostomy Safety Project (NTSP http://www.tracheostomy.org.uk/) has published multidisciplinary guidelines for the management of tracheostomy and laryngectomy airway emergencies (McGrath et al. 2012a). These algorithms describe a universal approach to managing tracheostomy emergencies, and are designed to be followed by first responders. Whilst these are strengths, the algorithms might be more suitable for wards than for ICU as they emphasise blockage over displacement, and capnography, while mentioned, is not emphasised. In June 2014, The National Confidential Enquiry into Patient Outcome and Death (NCEPOD http://www.ncepod.org.uk/trachy.htm ) will publish the results of a two-year long study of tracheostomy care on ICU and wards, aiming to identify any remediable care failings. Finally, the Global Tracheostomy Collaborative (http://globaltrach.org/) will be launched in Boston, USA and London, UK in 2014. This multidisciplinary, international, not-for-profit collaborative aims to improve patient care by sharing best practices, supporting education and adopting integrated multidisciplinary care.
We should be optimistic that in the next few years these considerable efforts will lead to better understanding of risk factors for complications associated with tracheostomies and, in the words of NTSP, lead to “improvements in infrastructure, competency and training, equipment provision, and communication.”
Human Factors
- Organisational Preparedness
Organisational preparedness involves organisations identifying predictable problems of airway management and putting in place solutions (e.g. equipment, staffing, procedures, training) that aid recognition and management of these risks and mitigate complications. NAP4 recorded that 20% of major airway complications occur outside theatres and the commonest events are failed intubation, accidental extubation and displacement of a tracheostomy.
An example is airway assessment to reduce unanticipated difficult intubation: 10% of ICU intubations require multiple attempts (Mort 2004), and life-threatening complications such as hypoxia, hypotension and cardiac arrest occur in up to 25% of cases (Leibowitz 2006). Organisational structures that make airway assessment routine on ICU (as in anaesthetic practice) and lead to adoption of clear airway strategies (Cook et al. 2011a) might be expected to reduce unanticipated difficulty and complications.
NAP4 recommended the adoption of an intubation checklist and the immediate availability of a difficult airway trolley. Previously, a 10-point checklist (including pre-oxygenation, fluid loading, rapid sequence intubation with ketamine or etomidate, capnography, vasopressors as required, early sedation and the presence or two operators) was shown to reduce complication rates by approximately one-third (Jaber et al. 2010). Senior supervision of intubation reduced complication rates fourfold (Schmidt et al. 2008).
Currently organisational preparedness is limited. A UK survey reported that 4% of patients admitted to ICU had been admitted for management of a primary airway problem, and 6.3% were predicted to have a difficult airway (Astin et al. 2012). Fewer than 1 in 5 of patients identified as 'at increased risk' had an individualised airway management plan in place. Action plans for unanticipated airway displacement were rarely available (tracheal tube displacement plans present in 7% of ICUs, tracheostomy displacement plans in 10% of ICUs), and often those available were anaesthesia guidelines (Henderson et al. 2004). Training and staffing were issues: only a small minority could describe the equipment available for emergency transtracheal access, and one-third of ICUs reported out of hours cover sometimes provided by junior doctors without basic anaesthesia competencies. An Australasian survey reported similar results (Husain et al. 2012).
Of further concern, recent changes in ICU management such as ventilating in the prone position, daily sedation holds and ventilation without sedation have the potential to increase the incidence of unanticipated extubations. ICU doctors are also increasingly from non-anaesthetic backgrounds. It is notable that no national critical care organisation has yet provided guidelines on managing airway complications on ICU. Whilst many national airway societies have published recommendations for managing anaesthetic airway difficulties, it is likely that underlying pathology, limited physiological reserve and the impracticality of waking the critically ill patient mean that airway crises in ICU require different solutions. National and European ICU organisations might usefully develop guidance.
All modern ICUs should be prepared to manage the airway complications that will occur in their unit. NAP4 made it clear that airway management on ICU can be complex, with rescue techniques being more likely to fail than in anaesthetic emergencies. Predefined protocols for intubation and unanticipated airway events and individualised airway strategies for high risk patients will facilitate planning and communication, and enable the appropriate equipment and staffing resources to be prepared. Training and audit should be in place. All form part of organisational preparedness.
- Individual Preparedness
A high proportion of the events reported to NAP4 occurred out of hours, when ICUs are more likely to be staffed by fewer and less experienced doctors (Leibowitz 2006), who may not have anaesthetic training (Astin et al. 2012; Husain et al. 2012).
NAP4 recommended that airway experts should be rapidly available for ICU doctors without advanced airway skills: the surveys cited above provide little reassurance.
Teaching Anaesthetic Non-Technical Skills (ANTS) has gained increasing prominence throughout anaesthetic and ICU practice (Flin and Patey 2011). It describes understanding the cognitive, interpersonal and social attributes of an individual to improve overall performance, reduce human error and enhance patient safety. ANTS comprises four key skill categories: situation awareness, decision-making, task management, and team working/leadership (Flin et al. 2008). NAP4 and other studies have directly linked a lack of these skills, especially situation awareness, to the contributing factors underlying critical airway incidents in ICU (Flin et al. 2013). Intubation checklists and pre-prepared airway strategies have been proposed to improve situational awareness during airway interventions on the ICU (Cook et al. 2011b, Gaba et al. 1995; Hales and Pronovost 2006).
In our experience junior staff can be trained in a relatively short period to provide a basic level of airway competency. Closely monitored airway experience in elective anaesthetic cases, workplace-based simulation training and formal induction courses provide suitable learning opportunities (Bristol Medical Simulation Centre). The resources required are minimal. Low fidelity scenarios have been shown to rapidly improve the ability of inexperienced doctors to perform basic medical interventions (Grober et al. 2004). Airway competency should not be assumed, and airway strategies should recognise available skills (Mulcaster et al. 2003): more than 50 intubations are required to achieve >90% success rate, with I in 5 trainees still requiring assistance after 80 supervised intubations (Konrad et al. 1998). Emphasising simpler airway management techniques (e.g. i-gel® (Intersurgical®, Wokingham, UK) (Wharton et al. 2008) or Supreme LMA™ (Teleflex Medical, San Diego, CA, USA) insertion (Howes et al. 2010) and use of limited airway rescue algorithms (Kelly et al. 2013)) offers leaner solutions. Some suggest that a competency-based assessment should be undertaken by all new ICU staff (Association of Anaesthetists of Great Britain & Ireland).
Those working on modern ICUs should:
• know their own airway skillset and limitations;
• know when and how to call for assistance;
• have knowledge of local equipment, standard operating procedures (SOPs) and, algorithms;
• engage in audit and training
All form part of individual preparedness.
Capnography
NAP4 reported that failure to use capnography contributed to >80% of airway-related deaths on ICU. The report recommended the use of continuous capnography for patients dependent on an artificial airway, and described this as the single change most likely to reduce complications and deaths (Cook et al. 2011b; Fischler et al. 2000). This recommendation was rapidly endorsed by national and European organisations (Association of Anaesthetists of Great Britain & Ireland; Thomas et al. 2012; European Board of Anaesthesiology 2011).
In 2003 a UK survey of capnography in adult ICUs focused on its use for confirming tracheal tube placement (Kannan and Manji 2003). One-quarter of units did not have a capnograph, and only one-fifth had one per bed. Capnography was used at intubation in only half of units which possessed one and routinely by only 1 in 4 units. Remarkably, half of respondents did not think that capnography should be mandatory for intubations outside the operating theatre. In 2010 Georgiou reported modest improvements: 25% ICUs used capnography for continuous monitoring during controlled ventilation, but 40% never used it (Georgiou et al. 2010). In paediatric practice capnography was used for all intubations in only 11% of ICUs (Cumming and McFadzean 2005). There is little published data on use of capnography in neonatal ICUs.
However, fatal avoidable airway errors do occur in such settings (Higgins 2013).
Surveys published after NAP4, but conducted before its final report, suggest further progress has been made. In 2012 a UK survey reported that 56% routinely used continuous waveform capnography for patients with artificial airways (Astin et al. 2012), and Husain from Australasia reported that capnography was used during tracheal intubation in 88% of ICUs and for continuous monitoring in 64% (Husain et al. 2012).
Capnography should be considered not only as a tool to confirm intubation, but (perhaps even more importantly) also as an airway and respiratory monitor. There is evidence that continuous capnography throughout a period of ventilatory support on adult ICU is becoming routine care in the UK (TM Cook, Unpublished data). Less is known about non-adult practice.
It is likely that the role of capnography in ICU can be developed further still. Opportunities for development and research include:
(i) making its use mandatory for patients dependent on an artificial airway;
(ii) ensuring that changes in adult practice reach paediatric and neonatal ICUs;
(iii) extending its use during non-invasive ventilation and sedation (Manifold et al. 2013);
(iv) exploring the diagnostic roles of qualitative and quantitative capnography trace analysis (e.g. assessing volume responsiveness (Young et al. 2013), lung recruitment manoeuvres (Tusman et al. 2014), monitoring bronchospasm (Yaron et al. 1996));
(v) exploring technical and non-technical methods to improve reliability of use of capnography (Hodges et al. 2012).
Videolaryngoscopy in ICU
The second commonest primary event leading to reports to NAP4 from ICU was failed intubation. Use of carefully selected videolaryngoscopy (VL) offers the potential to reduce intubation difficulty in general (Behringer and Kristensen 2011), including on ICU.
Videolaryngoscopes can be divided into three main groups (Niforopoulou et al. 2010):
1. Macintosh-blade shaped videolaryngoscopes
(e.g. C-MAC (Karl Storz, Tuttligen, Germany), McGrath MAC (Aircraft Medical, Edinburgh, UK), AP Venner with Macblade (Venner Medical, Dänischenhagen, Germany)) can be used for conventional direct laryngoscopy (DL) or for indirect VL. The distally placed camera enables the operator to ‘see around the corner’ and to achieve a wider angle of view than with DL (see Figure 1).
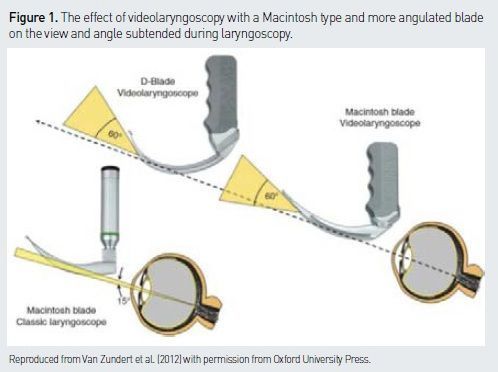
2. Angulated videolaryngoscopes
(e.g. Glidescope (Verathon, Bothell, WA, USA), McGrath 5 (Aircraft Medical, Edinburgh, UK), CMAC D blade ((Karl Storz, Tuttligen, Germany), AP Venner scope with ‘standard blade’ (Venner Medical, Dänischenhagen, Germany)) have more angulated blades than a standard Macintosh and only provide an indirect VL view of the larynx. They require the use of a stylet or a bougie.
3. Channelled videolaryngoscopes
(e.g. Airtraq (Prodol Meditec, Vizcaya, Spain), Pentax-Airway Scope (Pentax Medical, Hamburg, Germany), AP Venner with difficult airway blade ((Venner Medical, Dänischenhagen, Germany)) have angulated blades and a guiding channel that directs the tracheal tube towards the glottis opening. Use of VL improves the view at laryngoscopy (Behringer and Kristensen 2011), but the advantages of VL on ICU also include effects on teamwork, communication and situation awareness as much as on technical skills (Kelly and Cook 2014). Use of VL aids training of junior doctors (a supervising doctor can share the intubating doctor’s view of the larynx on the screen, give advice to assist intubation and usually enable the junior doctor to complete the intubation themselves), training of ICU nurses (enabling nurses to monitor the effect of cricoid pressure and adjust it as needed), improves management of the unanticipated difficult airway because a videolaryngoscope is immediately to hand, and improves team working and situation awareness as the screen enables all staff involved to see what is happening and anticipate the next steps (Kelly and Cook 2014).
An important advantage of VL based on a Macintosh blade is that it uses the same skills as DL, thereby reducing the need for dedicated VL training. We have a dedicated CMAC™ videolaryngoscope (Karl Storz, Culver City CA) on our ICU, and teach our trainees to use this as they would a standard MacIntosh blade. Initially the trainee is not allowed to see the VL screen (although the trainer watches this), but it is available if difficulty is encountered. In addition the intubation can be recorded for teaching after the event. A D-blade is immediately available when intubation is awkward or difficult. VL has become routine practice for all intubations on our ICU.
The overall impact of VL in the anaesthetic literature is confused due to marked heterogeneity in patient population, device studied, operator experience and confusion when manikin studies are included (Mihai et al. 2008). Whilst VL improves the ease of obtaining a view of the larynx, inserting a tracheal tube can be more difficult. Use of VLs on ICU is difficult to study for similar reasons, but is increasing in popularity (Mosier et al. 2013; De Jon et al. 2013; Lakticova et al. 2013). There is a need for randomised controlled trials (RCTs) of VL vs DL on ICU (Larsson and Dhonneur 2013): these need to be performed before VL use on ICU is so widespread that such a study is impractical. RCTs would help determine which devices are of greatest utility, and could study the impact of VL on both technical and human factors (Kelly and Cook 2014).
Conclusions
NAP4 showed that ICU can be a place of airway danger and that the quality of airway care falls short more often than in anaesthetic practice. This results in avoidable harm to patients. It is important the ICU community acknowledges this gap in quality of care and continues to work towards closing it. Understanding our patients’ needs, understanding the limitations of our technical and non-technical skills, using appropriate monitoring of the airway throughout the patient’s ICU stay and evaluating and embracing appropriate new technology will go a long way to closing this gap.
For full references, please email [email protected], visit the website at www.icu-management.orgor use the QR code at the top of the article.


















