ICU Management & Practice, Volume 23 - Issue 1, 2023
This article describes the application of existing and emerging biomarkers in the diagnosis and management of sepsis and pneumonia.
Introduction: The Need for Biomarkers in Sepsis
Sepsis is defined as a dysregulated host response to infection that results in life threatening organ dysfunction. It carries an estimated 30-day mortality rate of 24.4% and increases to 34.7% in patients who develop shock (Singer et al. 2016; Bauer et al. 2020). A main pillar in sepsis management is the early initiation of antibiotics. However, inappropriate use of broad-spectrum antibiotics can lead to antimicrobial resistance and even increase mortality (Kumar et al. 2009; Rhee et al. 2020; Teshome et al. 2019). Identification of the 30.6% to 56.4% of patients with culture negative sepsis could prevent these adverse outcomes through the implementation of antibiotic de-escalation, and even in those with positive cultures, it may be possible to reduce the duration of therapy (Gupta et al. 2016; Kethireddy et al. 2018; Nanna Panday et al. 2019). In addition, current clinical and laboratory-based scoring systems lack sensitivity and specificity to guide clinicians in triage decision making and prognostication (Churpek et al. 2017; Freund et al. 2017; Raith et al. 2017; Wang et al. 2022). To aid in these dilemmas, clinicians have turned to biomarkers, defined as molecules that can be objectively measured and evaluated as an indicator of underlying biological processes (Biomarkers Definitions Working Group 2001). The ideal biomarker in sepsis would aid in one or more of the following domains: 1) antibiotic initiation, and differentiation between bacterial infection, viral infection, and sterile inflammation; 2) antibiotic treatment duration; and 3) prognostication (Pierrakos et al. 2020). In this review we discuss these specific applications for the following existing and emerging biomarkers: procalcitonin, presepsin, pentraxin-3, and pancreatic stone proteins in sepsis. Finally, we discuss these biomarkers in comparison, as well as their multimodal application in sepsis.
Procalcitonin
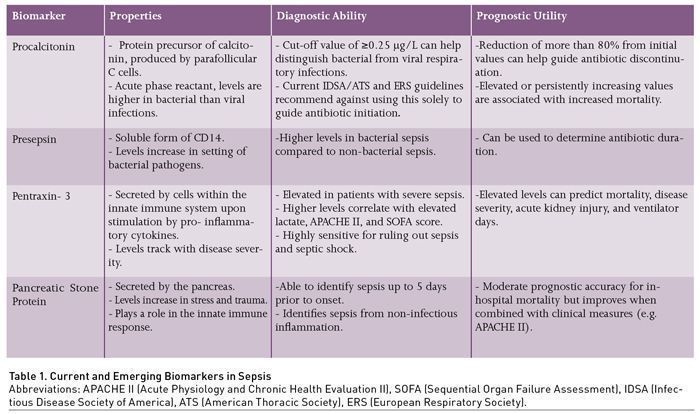
Procalcitonin (PCT) is a protein precursor of calcitonin produced by the parafollicular C cells of the thyroid glands (Table 1) (Becker et al. 2004). In response to inflammation, PCT synthesis behaves as an acute phase reactant and is activated in peripheral tissues such as the kidney and liver, followed by subsequent increases in serum levels, up to 100,000 times normal levels, in the setting of bacterial infection (Christ-Crain and Müller 2007). This increase tends to be specifically observed in response to bacteria, but less so with viruses. Bacterial toxins and host cytokines appear to increase PCT levels, whereas molecules produced in response to viruses attenuate PCT production (Becker et al. 2004; Christ-Crain and Müller 2007; Christ-Crain and Müller 2005). Given this property, PCT has been widely studied as a biomarker in sepsis, especially in relation to lower respiratory tract infections (LRTI). It has been evaluated in all aspects of biomarker application as previously described, which include guiding antibiotic initiation, determining antibiotic duration, and prognostication. Currently, its most relevant clinical utility is in guiding antibiotic duration, while its usefulness in other applications remains in debate. In current sepsis guidelines, it is recommended to use PCT, along with clinical data, to guide duration of therapy, and not for determining when to start therapy.
Diagnosis and Antibiotic Initiation
The use of PCT to guide antibiotic initiation has been studied in several randomised controlled clinical trials (RCT), with a majority in pulmonary infections and respiratory diseases that include chronic obstructive pulmonary disease (COPD) exacerbations. These trials used serial measurements of PCT levels to determine the initiation or discontinuation of antibiotics, compared with standard practice, at the time the RCTs were conducted. Results of these trials have been conflicting. For example, two of the larger trials, the ProHOSP and the ProACT trial produced different results. The ProHOSP trial showed that a PCT based algorithm significantly shortened the average number of antibiotic days (5.7 vs 8.7 days), for respiratory infection, whereas the ProACT trial did not show a meaningful difference (4.2 vs 4.3 days) (Schuetz et al. 2009; Huang et al. 2018). An argument supporting the ProHOSP trial is that the ProACT trial was conducted nearly a decade after the ProHOSP trial when standard practice had already incorporated shorter antibiotic durations. This can be seen in the difference in average antibiotic days between the two RCTs. Nonetheless, other clinical outcomes such as adverse events and infection relapse were not significantly different between the trial groups.
Additional areas of ambiguity include varying levels of acceptable thresholds for antibiotic initiation. A level of ≥0.25 μg/L has been used as a general cut-off for bacterial infections; however studies in respiratory infections have demonstrated that no specific value adequately predicted bacterial infection (Self et al. 2017; Musher and Thorner 2014; Schuetz et al. 2017). As a result, professional guidelines such as the surviving sepsis campaign, and pneumonia treatment guidelines from the Infectious Disease Society of America (IDSA)/American Thoracic Society (ATS), as well as the European Respiratory Society (ERS) have recommended against the reliance on PCT in guiding antibiotic initiation (Torres et al. 2017; Metlay et al. 2019; Kalil et al. 2016). Furthermore, while PCT can be specifically elevated in response to bacterial infection, false elevations can be observed in sterile inflammation. Examples include, but are not limited to pancreatitis, trauma, surgery, burns, and cardiac arrest (Mimoz et al. 1998; Annborn et al. 2013; Meisner et al. 1998; Kylänpää-Bäck et al. 2001).
Antibiotic Duration
Recent data support the use of PCT in guiding antibiotic duration. Three RCTs involving critically ill sepsis patients – the PRORATA, SAPS, and PROGRESS TRIALS – have shown that serial measurements, with decreasing PCT levels, can be used as a safe metric for antibiotic discontinuation in conjunction with best clinical judgment (Bouadma et al. 2010; de Jong et al. 2016; Kyriazopoulou et al. 2021). The trials used ≤0.5 μg/L or an 80% reduction from baseline level as a guide for antibiotic discontinuation. All three trials found a significantly shorter antibiotic duration with PCT-guided treatment. The PRORATA trial showed no significant difference in mortality and re-infection risk. However, in the SAPS and PROGRESS trials, significant mortality reductions at 28 days (also reduction in 1-year mortality for the SAPS trial) were observed. Furthermore, the PROGRESS trial, which was initially designed to assess the aetiology behind the mortality reduction observed in the SAPS trial, found reduction in long-term adverse outcomes with PCT-guided treatment such as acute kidney injury, and diarrhoea. However, colonisation by multidrug resistant organisms (MDRO) was similar between the two groups.
Prognostication
The prognostic value of PCT had been primarily evaluated in LRTIs and can be valuable in clinical practice when combined with other risk prediction models. Initial PCT elevations, and more so, up trending levels have been associated with increased mortality or treatment failure in patients with LRTI. These findings were observed in a meta-analysis of 14 trials, as well as in several observational studies (Kutz et al. 2015; Boussekey et al. 2006; Bloos et al. 2011). Much like in antibiotic initiation, no cut-off value for a single measurement had been specifically associated with poor outcomes. Furthermore, studies have reported conflicting results as to whether PCT levels are truly predictive of mortality (Ryoo et al. 2019). A possible approach may be to use serial measurements for prognostication, which can potentially carry more value as has been the case in guiding antibiotic duration.
Presepsin
Presepsin is an emerging immunological biomarker that is a soluble form of CD14. CD14 is a surface glycoprotein and member of the Toll-like receptors, expressed by macrophages and monocytes, with affinity to bacterial ligands such as lipopolysaccharide (Xiao et al. 2022). In the presence of bacterial pathogens, presepsin levels increase early, as it is a by-product of the innate immune response (Chenevier-Gobeaux et al. 2015). This is particularly advantageous in identifying bacterial sepsis early in the disease course (Leli et al. 2016; Wu et al. 2017). While it is currently not widely available, presepsin has been evaluated in guiding the diagnosis of bacterial sepsis, antibiotic duration, and sepsis prognosis.
Diagnosis of Bacterial Sepsis
Presepsin has the potential to distinguish bacterial infections from non-bacterial infections as a cause of sepsis, based on several observational studies. Higher presepsin levels have been associated with bacterial sepsis compared to non-bacterial sepsis, consistent with its underlying biology (Pugni et al. 2015; Masson et al. 2015; Liu et al. 2013; Endo et al. 2012). Threshold values indicative of bacterial sepsis were reported in the 600 ng/L range, with reported sensitivity of 87.8% and specificity of 81.3% (Endo et al. 2012). However, the range of values varies across studies, as well as its predictive value (Azim 2021). Therefore, at this time there are no guidelines or recommendations for an accepted discriminant value until further studies are conducted.
Antibiotic Duration
Much like PCT, presepsin has potential value for improving antibiotic stewardship and contributing to decisions to escalate or de-escalate antibiotic therapy. In an unrandomised, multicentre trial in China of 656 patients, a presepsin-guided treatment protocol was compared to standard of care (Xiao et al. 2022). Antibiotic therapy was discontinued when levels reached below 350 pg/mL or decreased by ≥80% from baseline. There were no significant differences in 28-day mortality, and the presepsin group had a significant reduction in antibiotic days compared to standard of care (14.54 vs 11.01 days). In contrast to decreasing levels, persistently elevated or rising presepsin concentration has been associated with inadequate treatment and persistent positive blood cultures (Masson et al. 2015; Liu et al. 2013; Endo et al. 2012; Azim 2021; Kweon et al. 2014). Therefore, in addition to antibiotic de-escalation, presepsin might be able to be used to escalate therapy when clinically appropriate.
Prognostication
Presepsin also has potential in prognostication in sepsis, such as predicting mortality, disease severity, and adverse outcomes. Initially elevated levels or persistently increasing levels have been associated with adverse outcomes across multiple studies (Masson et al. 2015; Kweon et al. 2014; Kim et al. 2017). These include the development of acute kidney injury, increased lengths of hospitalisation, increased ventilator days, increased vasopressor duration, decreased infection clearance, and mortality.
Overall, additional studies are needed to support presepsin’s potential clinical applications. Nonetheless, the current available data show a promising future for this biomarker in guiding sepsis management.
Pentraxin 3
Pentraxin 3 (PTX3) is involved in the innate immune response as it is produced by dendritic cells, monocytes, and macrophages (along with many others) upon stimulation of the pro-inflammatory cytokines TNFα and IL-1ß, or damage-associated molecular patterns (Magrini et al. 2016). PTX3 can then promote inflammation through complement activation and opsonisation but also attenuate the immune response by binding P-selectin on neutrophils, decreasing their recruitment to sites of inflammation (Bottazzi et al. 2010; Deban et al. 2010). PTX3 can be an ideal biomarker in sepsis as levels increase with disease severity, for example from systemic inflammatory response syndrome (SIRS) to septic shock (Muller et al. 2001).
Diagnostic Applications
As an acute phase protein, PTX3 can aide in clinical diagnosis of sepsis. In patients who presented to the emergency department (ED), PTX3 was found to be elevated in patients with severe sepsis compared to those without severe sepsis (median value 16.7 ng/mL vs 4.9 ng/mL, p < 0.001) and a cut-off value of 14.1 ng/mL was associated with a higher odds of having severe sepsis (odds ratio 6.77, 95% CI 3.64 – 12.59, p < 0.001) (Uusitalo-Seppala et al. 2013). In a study of 213 intensive care unit (ICU) patients with sepsis and septic shock PTX3 correlated well with higher lactate, the Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA) score. On day 1 of ICU care, a cut-off value of 5 ng/mL and 9 ng/mL for septic shock provided a sensitivity of 98 and 93% but only a specificity of 79 and 45% indicating that this can be a good test in ruling out these disease states (Hamed et al. 2017).
Prognostic Applications
Elevated levels of PTX3 have shown prognostic utility in predicting future organ failure and disease severity (Huttunen et al. 2011; Bastrup-Birk et al. 2013). Multiple studies have shown that elevated PTX3 levels were associated with worse mortality in patients with sepsis and septic shock (Uusitalo-Seppala et al. 2013; Bastrup-Birk et al. 2013; Chen et al. 2021; Jie et al. 2017; Perez-San Martin et al. 2020). A recent meta-analysis has confirmed the association of elevated PTX3 levels and mortality (hazard ratio 2.09; 95% CI 1.55 – 2.81 p < 0.001, AUC 0.73; 95% CI 0.7 – 0.77 p < 0.001); however there was high heterogeneity in the studies analysed and the optimal cut-off value for mortality prediction remains uncertain (Wang et al. 2022).
In summary, PTX3 appears to be a useful biomarker in predicting disease severity and mortality. However, more well balanced and larger studies should be performed before any definitive conclusions can be made.
Pancreatic Stone Protein
Pancreatic stone protein (PSP) is a molecule secreted primarily by the pancreas, but also in other parenchymal cells during the stress response (Terazono et al. 1988). It was formerly identified as two separate molecules, lithostathine and regenerating protein 1. However, they were later found to be similar and eventually renamed PSP (Terazono et al. 1988). The various functions of PSP remain under investigation and continue to be unravelled. However, it has been recognised to play a role in inflammation and the innate immune response, such as activation of polymorphonuclear cells (Eggimann et al. 2019). Concentrations of PSP have been observed to increase in response to stress and trauma, especially when the inciting event leads to infection and sepsis (Reding et al. 2017). Given this property, PSP has been studied both as a diagnostic biomarker to identify sepsis, and as a prognostic marker to evaluate treatment response.
Diagnosis of Sepsis
The utility of PSP comes from its potential for the early identification of sepsis, even before the onset of clinical signs and symptoms. Serial serum measurements of PSP increase leading up to nosocomial sepsis with elevated levels being detected as early as five days prior (Pugin et al. 2021). Similarly, in a study involving burn patients, PSP showed a steep rise 72 hours prior to the development of sepsis and was able to differentiate between septic and non-septic patients (Klein et al. 2021). At the point of admission, PSP can be highly accurate in the diagnosis of sepsis with infection compared to patients without infection (AUC 0.839; 95% CI 0.773 – 0.904), and those with sepsis compared to a non-infectious SIRS (AUC 0.91; 95% CI 0.86 – 0.96) (Garcia de Guadiana-Romualdo et al. 2017; Llewelyn et al. 2013). A recent systematic review and patient level meta-analysis showed that PSP has moderate diagnostic accuracy for the discrimination of septic vs non-septic patients with a specificity of 0.83 and sensitivity of 0.66 using a cut-off of value of 44.18 ng/mL (Prazak et al. 2021). Finally, the implementation of a PSP decision tree model in patients with suspected sepsis led to a reduction in healthcare costs in the ED and ICU compared to the standard of care. This indicates that PSP has utility in not just sepsis diagnosis but also in healthcare cost reduction (Schneider et al. 2022).
Prognostication
In a prospective cohort study, Que et al. (2012) studied whether PSP drawn within 24 hours of admission could predict in-hospital mortality. Median levels of PSP were elevated in patients who died, compared to those that survived (397 ng/mL vs. 216.1 ng/mL, p < 0.02). However, the overall prognostic accuracy was moderate at best and the prognostic accuracy for in-hospital mortality was poor (AUC 0.65; 95% CI 0.51 – 0.80). The poor prognostic accuracy of PSP can be improved with the addition of a clinical severity score measure such as the APACHE II or the Simplified Acute Physiology Score II (SAPS II) (Que et al. 2015). Further research involving large cohorts of patients with sepsis and septic shock are needed to further clarify the prognostic implications of PSP.
Comparison of PCT, Presepsin, PTX3, and PSP
Since PCT is one of the earlier and most studied biomarkers in sepsis and LRTI, its utility has been compared to the newer and emerging biomarkers. Liu et al. (2013) examined the prognostic accuracy of presepsin compared to PCT in the context of patients presenting to the ED with SIRS. They found that using a cut-off value of 317 pg/mL for presepsin produced a sensitivity of 70.8% and specificity of 85.8% compared to a sensitivity of 60% and specificity of 77.7% for PCT (cut-off value of 0.25 ng/mL). These findings are divergent from a recent prospective observational study of 420 patients with non-infectious organ failure, sepsis, and septic shock where PCT (cut-off 0.51 ng/mL, sensitivity 75.5%, specificity 93%) was marginally better at differentiating patients with sepsis compared to non-infectious organ failure than presepsin (cut-off 582 pg/mL, sensitivity 70.1%, specificity 89.4%) (Lee et al. 2022).
PTX3 has shown comparable accuracy for sensitivity and specificity for the diagnosis of septic shock compared to PCT (PTX3; AUC 0.73, 95% CI 0.66 – 0.80 vs PCT; AUC 0.73, 95% CI 0.65 – 0.80) but is slightly worse when it comes to the diagnosis of sepsis (PTX3; AUC 0.68, 95% CI 0.58 – 0.78 vs PCT; AUC 0.79, 95% CI 0.70 – 0.88) (Chen et al. 2021). PTX3 and PCT perform equally well for 28-day mortality prediction in patients with a diagnosis of sepsis but ultimately perform worse compared to clinical scores such as the APACHE II (Hu et al. 2018).
When compared to PCT, PSP performs equally well in distinguishing patients with sepsis from those without (Pugin et al. 2021). A patient level meta-analysis found that both PCT and PSP perform equally well in diagnosing hospitalised patients with an infection, with superior accuracy when both biomarkers were combined (Prazak et al. 2021).
Multi-Marker Application in Sepsis
While existing and emerging biomarkers hold promise in guiding sepsis management, no biomarker is perfect and yields variable predictive capabilities in its diagnostic and prognostic utilities. As with every clinical tool, biomarkers play a role as adjuncts to broader clinical assessments in helping us predict clinical outcomes. Combining biomarkers together with existing predictors, such as the SOFA score, may have promise in improving predictive performance in both the diagnostic and prognostic arenas.
Mearelli et al. (2018) evaluated the performance of predictive models incorporating multiple serum biomarkers including PCT, phospholipase A2 group IIA (sPLA2GIIA), presepsin, soluble interleukin-2 receptor α (sCD25), and soluble triggering receptor expressed on myeloid cell (sTREM-1) to determine whether patients had infectious vs non-infectious SIRS. The model was trained on 947 adults presenting to the ED of five Italian university hospitals and validated in 185 adults. The patients were stratified into low, intermediate, and high probability of infection based on a predictive nomogram, and the models were assessed in each group. In the intermediate probability group, the multi-marker model had the highest predictive capability in identifying infectious SIRS, yielding an area under the receiver operating characteristic curve (AUROC) of 0.85 (95% confidence interval 0.778-0.923) for patients with sepsis or septic shock. The individual biomarkers showed lower AUROCs ranging from 0.56 to 0.77 (Mearelli et al. 2018).
A combination of biomarkers and clinical features was also studied in predicting prognosis in patients with sepsis. Kim et. al (2017) evaluated a combination of SOFA score, white blood cell count, C-reactive protein, PCT, presepsin, galectin-3, soluble suppression of tumourigenicity 2 (sST2) in 157 patients with either sepsis or septic shock. The predictive performance of the model improved with stepwise addition of each biomarker in addition to the SOFA score. The multi-marker approach with SOFA showed an AUROC of 0.769 (95% confidence interval 0.695 – 0.833) vs SOFA score alone with an AUROC of 0.615 (95% confidence interval 0.535 – 0.692). Although this study was limited by its small sample size, the initial results pave a way for future research to re-evaluate the multi-marker approach further.
Conclusion and Future Directions
As the role of biomarkers in sepsis continues to evolve, current evidence lends strong support for the diagnostic and prognostic utility of PCT, presepsin, PTX3, PSP, and their combination with other existing clinical predictors in patients with sepsis and septic shock. In assessing the literature, it is key to focus on whether data are being used for sepsis diagnosis, prognosis, or to determine duration of therapy. Further research involving these biomarkers should focus on determining the optimal cut-off levels for the diagnosis of sepsis, and the development of clinical decision tools that incorporates these biomarkers to aid clinicians in predicting and improving outcomes in these patients.
Conflict of Interest
Dr Di Pan and Dr William Whalen have no conflict of interest to declare. Dr Michael S Niederman has been a consultant to Thermo Fisher, but has no other relevant disclosures.
References:
Annborn M, Dankiewicz J, Erlinge D et al. (2013) Procalcitonin after cardiac arrest - an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation. 84(6):782-7.
Azim A (2021) Presepsin: A Promising Biomarker for Sepsis. Indian J Crit Care Med. 25(2):117-118.
Bastrup-Birk S, Skjoedt MO, Munthe-Fog L et al. (2013) Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 8(9):e73119.
Bauer M, Gerlach H, Vogelmann T et al. (2020) Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 24(1):239.
Becker KL, Nylén ES, White JC et al. (2004) Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 89(4):1512-25.
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 69(3):89-95.
Bloos F, Marshall JC, Dellinger RP et al. (2011) Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 15(2):R88.
Bottazzi B, Doni A, Garlanda C, Mantovani A (2010) An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 28:157-83.
Bouadma L, Luyt CE, Tubach F et al. (2010) Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 375(9713):463-74.
Boussekey N, Leroy O, Alfandari S et al. (2006) Devos P, Georges H, Guery B. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 32(3):469-72.
Chen H, Li T, Yan S, et al. (2021) Pentraxin-3 Is a Strong Biomarker of Sepsis Severity Identification and Predictor of 90-Day Mortality in Intensive Care Units via Sepsis 3.0 Definitions. Diagnostics (Basel). 11(10).
Chenevier-Gobeaux C, Borderie D, Weiss N et al. (2015) Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clinica Chimica Acta. 450:97-103.
Christ-Crain M, Müller B (2007) Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 30(3):556-73.
Christ-Crain M, Müller B (2005) Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med Wkly. 135(31-32):451-60.
Churpek MM, Snyder A, Han X et al. (2017) Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am J Respir Crit Care Med. 195(7):906-911.
Deban L, Russo RC, Sironi M et al. (2010) Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 11(4):328-34.
de Jong E, van Oers JA, Beishuizen A et al. (2016) Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. The Lancet Infectious Diseases. 16(7):819-827.
Eggimann P, Que Y-A, Rebeaud F (2019) Measurement of pancreatic stone protein in the identification and management of sepsis. Biomarkers in medicine. 13(02):135-145.
Endo S, Suzuki Y, Takahashi G et al. (2012) Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy. 18(6):891-897.
Freund Y, Lemachatti N, Krastinova E et al. (2017) Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA. 317(3):301-308.
Garcia de Guadiana-Romualdo L, Berger M, Jimenez-Santos E et al. (2017) Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department. Eur J Clin Invest. 47(4):297-304.
Gupta S, Sakhuja A, Kumar G et al. (2016) Culture-Negative Severe Sepsis: Nationwide Trends and Outcomes. Chest. 150(6):1251-1259.
Hamed S, Behnes M, Pauly D et al. (2017) Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. 17(1):554.
Hu C, Zhou Y, Liu C, Kang Y (2018) Pentraxin-3, procalcitonin and lactate as prognostic markers in patients with sepsis and septic shock. Oncotarget. 9(4):5125-5136.
Huang DT, Yealy DM, Filbin MR et al. (2018) Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N Engl J Med. 379(3):236-249.
Huttunen R, Hurme M, Aittoniemi J et al. (2011) High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS One. 6(3):e17653.
Jie H, Li Y, Pu X, Ye J (2017) Pentraxin 3, a Predicator for 28-Day Mortality in Patients With Septic Shock. Am J Med Sci. 353(3):242-246.
Kalil AC, Metersky ML, Klompas M et al. (2016) Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical Infectious Diseases. 63(5):e61-e111.
Kethireddy S, Bilgili B, Sees A et al. (2018) Culture-Negative Septic Shock Compared With Culture-Positive Septic Shock: A Retrospective Cohort Study. Crit Care Med. 46(4):506-512.
Kim H, Hur M, Moon HW et al. (2017) Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann Intensive Care. 7(1):27.
Klein HJ, Niggemann P, Buehler PK et al. (2021) Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann Surg. 274(6):e1179-e1186.
Kumar A, Ellis P, Arabi Y et al. (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 136(5):1237-1248.
Kutz A, Briel M, Christ-Crain M et al. (2015) Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Crit Care. 19(1):74.
Kweon OJ, Choi J-H, Park SK, Park AJ (2014) Usefulness of presepsin (sCD14 subtype) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. Journal of Critical Care. 29(6):965-970.
Kylänpää-Bäck ML, Takala A, Kemppainen EA et al. (2001) Procalcitonin, soluble interleukin-2 receptor, and soluble E-selectin in predicting the severity of acute pancreatitis. Crit Care Med. 29(1):63-9.
Kyriazopoulou E, Liaskou-Antoniou L, Adamis G et al. (2021) Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial. Am J Respir Crit Care Med. 203(2):202-210.
Lee S, Song J, Park DW et al. (2022) Diagnostic and prognostic value of presepsin and procalcitonin in non-infectious organ failure, sepsis, and septic shock: a prospective observational study according to the Sepsis-3 definitions. BMC Infect Dis. 22(1):8.
Leli C, Ferranti M, Marrano U et al. (2016) Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. Journal of Medical Microbiology. 65(8):713-719.
Liu B, Chen Y-X, Yin Q et al. (2013) Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Critical Care. 17(5):R244.
Liu B, Chen YX, Yin Q et al. (2013) Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit Care. 17(5):R244.
Llewelyn MJ, Berger M, Gregory M et al. (2013) Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit Care. 17(2):R60.
Magrini E, Mantovani A, Garlanda C (2016) The Dual Complexity of PTX3 in Health and Disease: A Balancing Act? Trends Mol Med. 22(6):497-510.
Masson S, Caironi P, Fanizza C et al. (2015) Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Medicine. 41(1):12-20.
Mearelli F, Fiotti N, Giansante C et al. (2018) Derivation and Validation of a Biomarker-Based Clinical Algorithm to Rule Out Sepsis From Noninfectious Systemic Inflammatory Response Syndrome at Emergency Department Admission: A Multicenter Prospective Study. Crit Care Med. 46(9):1421-1429.
Meisner M, Tschaikowsky K, Hutzler A et al. (1998) Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 24(7):680-4.
Metlay JP, Waterer GW, Long AC et al. (2019) Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. American Journal of Respiratory and Critical Care Medicine. 200(7):e45-e67.
Mimoz O, Benoist JF, Edouard AR et al. (1998) Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med. 24(2):185-8.
Muller B, Peri G, Doni A et al. (2001) Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 29(7):1404-7.
Musher DM, Thorner AR (2014) Community-acquired pneumonia. N Engl J Med. 371(17):1619-28.
Nannan Panday RS, Lammers EMJ, Alam N, Nanayakkara PWB (2019) An overview of positive cultures and clinical outcomes in septic patients: a sub-analysis of the Prehospital Antibiotics Against Sepsis (PHANTASi) trial. Crit Care. 23(1):182.
Perez-San Martin S, Suberviola B, Garcia-Unzueta MT et al. (2020) Prognostic value of plasma pentraxin 3 levels in patients with septic shock admitted to intensive care. PLoS One. 15(12):e0243849.
Pierrakos C, Velissaris D, Bisdorff M et al. (2020) Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care. 24(1):287.
Prazak J, Irincheeva I, Llewelyn MJ et al. (2021) Accuracy of pancreatic stone protein for the diagnosis of infection in hospitalized adults: a systematic review and individual patient level meta-analysis. Crit Care. 25(1):182.
Pugin J, Daix T, Pagani JL et al. (2021) Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: a prospective multicentric study. Crit Care. 25(1):151.
Pugni L, Pietrasanta C, Milani S et al. (2015) Presepsin (soluble CD14 subtype): reference ranges of a new sepsis marker in term and preterm neonates. PloS one. 10(12):e0146020.
Que YA, Delodder F, Guessous I et al. (2012) Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit Care. 16(4):R114.
Que YA, Guessous I, Dupuis-Lozeron E et al. (2015) Prognostication of Mortality in Critically Ill Patients With Severe Infections. Chest. 148(3):674-682.
Raith EP, Udy AA, Bailey M et al. (2017) Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 317(3):290-300.
Reding T, Palmiere C, Pazhepurackel C et al. (2017) The pancreas responds to remote damage and systemic stress by secretion of the pancreatic secretory proteins PSP/regI and PAP/regIII. Oncotarget. 8(18):30162-30174.
Rhee C, Kadri SS, Dekker JP et al. (2020) Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated With Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw Open. 3(4):e202899.
Ryoo SM, Han KS, Ahn S et al. (2019) The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: A multicenter prospective registry-based observational study. Scientific Reports. 9(1):6579.
Schneider JE, Dick K, Cooper JT, Chami N (2022) Pancreatic stone protein point-of-care testing can reduce healthcare expenditure in sepsis. Health Econ Rev. 12(1):39.
Schuetz P, Christ-Crain M, Thomann R et al. (2009) Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 302(10):1059-66.
Schuetz P, Wirz Y, Sager R et al. (2017) Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 10(10):Cd007498.
Self WH, Balk RA, Grijalva CG et al. (2017) Procalcitonin as a Marker of Etiology in Adults Hospitalized With Community-Acquired Pneumonia. Clin Infect Dis. 65(2):183-190.
Singer M, Deutschman CS, Seymour CW et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 315(8):801-10.
Terazono K, Yamamoto H, Takasawa S et al. (1988) A novel gene activated in regenerating islets. Journal of Biological Chemistry. 263(5):2111-2114.
Teshome BF, Vouri SM, Hampton N et al. (2019) Duration of Exposure to Antipseudomonal beta-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy. 39(3):261-270.
Torres A, Niederman MS, Chastre J et al. (2017) International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. Sep 50(3).
Uusitalo-Seppala R, Huttunen R, Aittoniemi J et al. (2013) Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One. 8(1):e53661.
Wang C, Xu R, Zeng Y et al. (2022) A comparison of qSOFA, SIRS and NEWS in predicting the accuracy of mortality in patients with suspected sepsis: A meta-analysis. PLoS One. 17(4):e0266755.
Wang G, Jiang C, Fang J et al. (2022) Pentraxin-3 as a predictive marker of mortality in sepsis: an updated systematic review and meta-analysis. Crit Care. 26(1):167.
Wu C-C, Hung C-J, Lin S-Y et al. (2017) Treadmill exercise alleviated prenatal buprenorphine exposure-induced depression in rats. Neurochemistry International. 110:91-100.
Xiao H, Wang G, Wang Y et al. (2022) Potential Value of Presepsin Guidance in Shortening Antibiotic Therapy in Septic Patients: a Multicenter, Prospective Cohort Trial. Shock. 57(1).






















