HealthManagement, Volume 20 - Issue 6, 2020
High dose biotin supplementation is most common among females and may cause faulty immunoassay results that delay diagnosis and treatment of serious medical conditions.
Key Points
- Biotin, also known as Vitamin B7, is an essential micronutrient that widely functions in the metabolic processing of amino acids, fats, and carbohydrates.
- The use of biotin supplementation has been disproportionately reported by women.
- Interference of biotin on troponin testing may lead to underdiagnosis of cardiovascular disease that disproportionately affects women.
- Further education for patients in regards biased immunoassay results should take place to ensure prompt diagnosis and treatment of serious health conditions.
Introduction
Evidence for the effects of biotin supplementation on laboratory testing has been increasingly identified as a possible source of bias between correlation of clinical symptoms and laboratory test values. The use of biotin supplementation has been disproportionately reported by women in the United States. There may be implications for women due to sex and gender differences in diagnosis and treatment of certain conditions that are managed based on the results of immunoassays which are adversely affected by high serum biotin concentrations.
Biotin
Biotin, also known as Vitamin B7, is an essential micronutrient that widely functions in the metabolic processing of amino acids, fats, and carbohydrates. The daily recommended dose of biotin for adults is 30 mcg which can be obtained from a regular, balanced diet. Signs of biotin deficiency include rashes, brittle nails, and loss of hair, and as such biotin supplementation has been marketed as a preventative measure for these conditions (Office of Dietary Supplements 2017). In the United States, 7.7% of adults self-reported the use of high dose biotin supplementation ranging from 1,000 to 50,000 mcg per day. Of this subset, 79.2% were women and 20.8% were men (Katzman et al. 2018).
Many widely used immunoassays utilise the biotin-streptavidin binding as a method of capturing the analyte. High serum concentrations of biotin can lead to assay interference (Gifford et al. 2019). Multiple assays, in particular high sensitivity troponin T (hs-TnT) and thyroid stimulating hormone (TSH), have been reportedly affected at serum biotin levels above 20 ng/mL (Nguyen et al. 2020). The resultant interference has affected results of tests in the form of falsely elevated or decreased values. In conjunction with clinical symptomatology and exam findings, a trend of lab errors secondary to biotin interference has the potential to adversely impact the diagnosis and treatment of female patients due to higher rates of exogenous supplementation.
Troponin
Cardiovascular disease is the leading cause of morbidity and mortality in both men and women in the United States. Traditionally viewed as a men’s disease, cardiovascular disease is curiously more prevalent in women. While major risk factors like hypertension and diabetes affect both men and women, evidence suggests that female-specific risk factors, such as pregnancy and early menopause, exert significant influence on the impact of traditional risk factors on cardiovascular disease severity (Appelman et al. 2015). Sex based differences in clinical presentation of acute cardiac diseases, including myocardial infarction, have also been implicated as significant challenges to risk stratification. This has been most notably evident in female patients because they are more likely than men to present with atypical symptoms that may cause acute cardiovascular disease to be missed (Saeed et al. 2017). As such, women are prone to less aggressive treatment compared to their male counterparts for the similar clinical presentations.
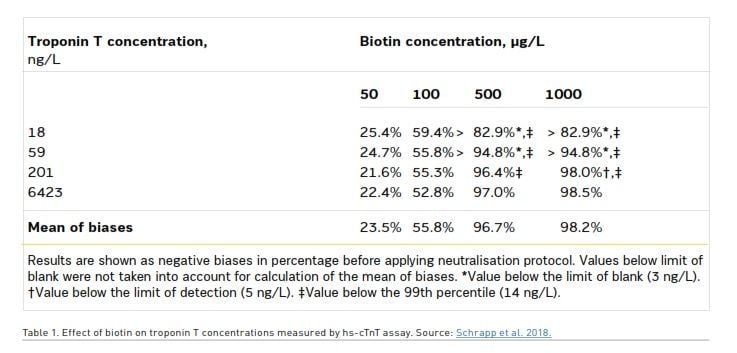
As the diagnosis of acute cardiovascular disease depends on evidence of myocardial injury detected by hsTnT assays, interference of biotin on troponin testing may lead to an even further underdiagnosis of cardiovascular disease that disproportionately affects women. In 2017, the FDA was notified about the death of a female patient who reported taking high dose biotin supplementation that died after a falsely low troponin level led to misdiagnosis. Her death was attributed to lab error secondary to biotin interference (Center for Devices and Radiological Health 2019). Serum biotin concentrations greater than 500 mcg/L have been linked to an over 95% decrease in troponin levels (Trambas et al. 2018). Interestingly, the quantitative troponin amount in a sample has been reported to not confer a direct correlation with the interference of biotin. Table 1 shows the initial absolute value of troponin demonstrating a mean negative bias that increases with higher levels of biotin, 23.5%, 55.8%, 96.7%, 98.2% at 50, 100, 500, 1000 mcg/L of biotin, respectively.
Of note, at the highest levels of biotin, 500 and 1000 mcg/L, the lower range of starting troponin levels of 18 and 59 mcg/L were reduced to the extent that a value to determine bias was unable to be recorded at the assay’s 3 ng/L threshold for detection (Schrapp et al. 2018). Thus, it cannot be excluded that patients taking high dose biotin supplements presenting with clinically significant troponin elevations below 60 mcg/L may be missed on the initial assay. The negative bias of troponin levels with high dose biotin supplementation, combined with the already widely observed sex-based differences in acute cardiovascular disease presentations and risk stratification, further elucidates the need for a heightened clinical suspicion for patients whose laboratory test values do not correlate with the clinical presentation. The most at risk patients for this bias and potential undermanagement of acute cardiac disease are women.
Thyroid Hormone
The rates of thyroid dysfunction between the sexes has been shown to be significantly different. In one study the prevalence of hyperthyroidism was 2.5% in females and 0.6% in males, and hypothyroidism 4.8% and 0.9% respectively (Bjoro et al. 2000). The onset of thyroid dysfunction is usually insidious. Before the introduction of modern laboratory testing, thyroid dysfunction was suggested by a number of clinical findings, including basal metabolic rate, heart rate, total serum cholesterol, reflex time, and creatinine kinase. A screening TSH is the single best test for thyroid dysfunction, supported by Free T3 and Free T4 (Garber et al. 2012). Interference threshold for TSH, Free T3 and Free T4 (x103 pg/mL) were 25, 70, and 100 respectively (Mrosewski et al. 2020). High dose biotin supplementation led to falsely high FT3 and FT4, and falsely low TSH (Trambas et al. 2018). Clinicians must be aware of the effects of biotin on immunoassays, and correlate test results with clinical evidence.
Of particular concern to women are the effects of thyroid dysfunction on the developing foetus during pregnancy. Children born to mothers with gestational hypothyroidism score significantly lower on numerous areas of neuropsychological testing, including intelligence, attention, reading ability, visual motor performance, and language. Maternal hyperthyroidism during gestation has been associated with an increased risk of spontaneous abortion, preterm delivery, low birth weight, and stillbirth. In addition to foetal complications, women with gestational hyperthyroidism are themselves at an increased risk for developing CHF, thyroid storm, and preeclampsia (Karassas et al. 2015). Prompt identification and treatment of these conditions during pregnancy is essential. Foetal cognitive deficits can develop in the first trimester if maternal hypothyroidism is inadequately managed. Maternal thyrotoxicosis is treated most often with methimazole or propylthiouracil during the first three months of pregnancy, both of which are associated with teratogenic effects and make reliable laboratory diagnosis essential (Barbour 2017). The clinician that is aware of maternal biotin supplementation can improve identification and management of these conditions.
Conclusion
The growing body of evidence suggesting biotin interference on numerous immunoassays further emphasises the importance of recognising sex-based differences in medical diagnosis and management. Nutritional supplements are becoming increasingly popular, but their regulation and indications are not yet widely accepted. Evidence that high dose biotin supplementation improves hair, skin, and nail growth is limited. We want to understate the fact that regular dietary and standard multivitamin amounts of biotin do not cause an elevation of biotin levels to the point that would cause interference with immunoassays or that there is any effect on pathophysiology of diseases. While some practitioners are privy to the possibility of erroneous low laboratory test results secondary to high dose biotin supplementation, it should be evident that further education for patients in regards biased immunoassay results should take place to ensure prompt diagnosis and treatment of serious health conditions (Bowen 2017). Our goal is to educate the medical community about the importance of documenting and reporting supplement use of patients to correlate with clinical presentation in the event of suspected lab error secondary to biotin interference.
Conflict of Interest
None.
References:
Appelman Y et al. (2015) Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis, 241(1): 211-218.
Barbour L (2017) Thyroid Disease & Pregnancy [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Department of Health and Human Services. Available from niddk.nih.gov/health-information/endocrine-diseases/pregnancy-thyroid-disease#hypothyroidism
Bjoro T, Holmen J, Krüger O et al. (2000) Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). European Journal of Endocrinology, 143(5):639-47.
Bowen R et al. (2019) Biotin interference: Underrecognized patient safety risk in laboratory testing. Clin Biochem, 74:1-11.
Center for Devices and Radiological Health. UPDATE: The FDA Warns that Biotin May Interfere with Lab Tests. (2019) U.S. Food and Drug Administration. Available from fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
Garber JR, Cobin RH, Gharib H et al. for the American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults KA (2012) Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 1;22(12):1200-35.
Gifford JL, de Koning L, Sadrzadeh SH (2019) Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clinical biochemistry, 1;65:61-3.
Katzman BM et al. (2018) Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin. Biochem, 60:11-16.
Krassas G, Karras SN, Pontikides N (2015) Thyroid diseases during pregnancy: a number of important issues. Hormones, 1;14(1):59-69.
Mrosewski I, Urbank M, Stauch T, Switkowski R (2020) Interference From High-Dose Biotin Intake in Immunoassays for Potentially Time-Critical Analytes by Roche: Evaluation of a Countermeasure for Worst-Case Scenarios. Archives of Pathology & Laboratory Medicine.
Nguyen KQN, et al. (2020) Assessment of Risk for Interference by Circulating Biotin in Samples Received for High Sensitivity Troponin-T, Thyrotropin, and for Prostate Specific Antigen Testing by Immunoassays. Clinical laboratory, 66(1).
Office of Dietary Supplements – Biotin. (2017) NIH Office of Dietary Supplements. U.S. Department of Health and Human Services; Available from ods.od.nih.gov/factsheets/Biotin-Consumer/
Saeed A, Kampangkaew J, Nambi V (2017) Prevention of Cardiovascular Disease in Women. Methodist Debakey Cardiovasc J, 13(4):185-192.
Schrapp A et al. (2018) Biotin and high-sensitivity cardiac troponin T assay. Biochemia medica, 28(3):523-527.
Trambas C et al. (2018) Characterization of the scope and magnitude of biotin interference in susceptible Roche Elecsys competitive and sandwich immunoassays. Ann Clin Biochem, 55:205–15.


















