HealthManagement, Volume 17 - Issue 5, 2017
An overview on the role of Health Technology Assessment (HTA) in healthcare
Prof. Giuseppe Lippi speaks with HealthManagement about his views on Health Technology Medicine in laboratory medicine and how it's improving the quality of care.
What do you think accredited scientific organisations can do to lend credibility to/increase confidence in HTAs?
The use of LEAN and HTA management is still largely insufficient in laboratory medicine compared to other medical fields. There are probably many issues that have contributed to such a poor diffusion, including lack of knowledge about the existence of these relatively innovative tools, the ongoing reorganisation of laboratory services driven by scale economy rather than by analytical efficiency and clinical efficacy, the lack of validated regulation for establishing an HTA process, as well as the strong - typically human - resistance against changes. The role of accredited scientific organisations is pivotal for increasing confidence in HTA , by disseminating the knowledge and demonstrating that HTA may be beneficial not only for sustaining laboratory economy, but also for improving laboratory organisation, clinical outcomes and patient safety.
Can HTA take the place of hands-on 'human' examination into lab processes?
Of course not. The role of HTA is to help in deciding whether a clinical test is generally cost-effective, but is not aimed to replace human examination of lab processes. To put it simply, what may work in a certain scenario, is not necessarily suitable for another. Esoteric testing and “-omics” techniques are paradigmatic examples. Due to ongoing restriction of healthcare funding, specialised diagnostic testing is becoming unsustainable in the single laboratory, but may find its most suitable place within a network of clinical laboratories, where the single facilities can specialise in one technology or medical field. This can also be seen as “scale economy”, but should be driven by laboratory professionals and not by policymakers or hospital administrators.
What do you think is necessary to make clinicians comfortable with HTAs for lab processes?
The clinical-laboratory interface is often a critical issue in laboratory medicine. Many clinicians find it difficult to understand that cost-containment policies are creating considerable barriers to innovation or, occasionally, to maintaining conventional diagnostic panels unaltered. Communication between laboratory professionals and clinicians needs to be improved and, especially, clinicians should consider that laboratory diagnostics do not come free. The most reliable solution is to further promote appropriateness, since what can be saved by reducing inappropriate or unjustified tests, can then be reinvested for introducing innovative technologies.
What are some of the challenges when it comes to sustainable diagnostic tests and how can they be overcome?
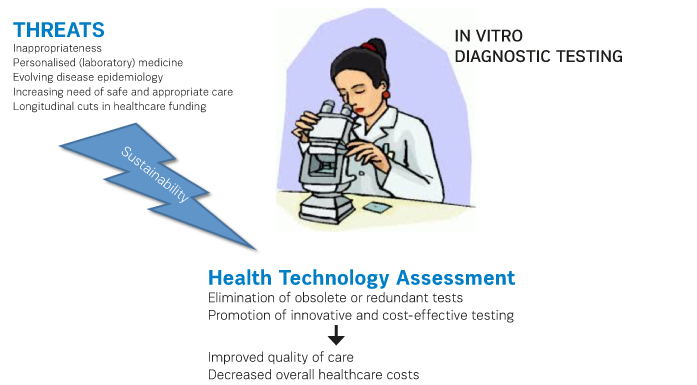
The sustainability of diagnostic testing is challenged by many issues. These include
- The
still excessively high burden of inappropriateness;
- The
increasing role of personalised (laboratory) medicine, which requires using
highly specific tests for dissecting genotypes, phenotypes and predicting
individual therapeutic response;
- The
evolving disease epidemiology, which is also recently sustained by increasing
immigration of populations from third-world to industrialised countries;
- The
increasing need of safe and appropriate care perceived by the patients,
worldwide;
- Longitudinal
cuts in healthcare funding, which do not specifically target cost-ineffective
activities. The solution of all these problems entails a cutting-edge approach,
based on renewed partnership among healthcare professionals and/ or scientific
organisations, citizens representatives, health and social-sanitary representatives,
commercial and non-profit partners and university delegates.
 Can
you please elaborate on some of the major drawbacks?
Can
you please elaborate on some of the major drawbacks?
The major drawbacks of HTA include the still scarce popularity (at least in laboratory medicine), the potential conflict with personalised (laboratory) medicine (i.e. the "one test fits all” paradigm is no longer valid, so that the outcome of HTA may not be generalisable in all patients), the lack of universal agreement about rules and regulations, as well as the potential conflict of interest of members of HTA teams.
How can we use HTA for sustainability of the healthcare system?
The outcome of many HTA analyses will be indeed valuable for the sustainability of the healthcare system, provided that the right approach will then be used in the right context. In general, HTA may help identifying obsolete or redundant testing, but may also promote the introduction of innovative tests, which can ultimately help decrease the overall healthcare expenditure. Predicting the development of certain pathologies within an appropriate time-frame and the therapeutic response will both generate a net benefit for the entire healthcare industry, since the incremental costs due to introduction of new tests can be completely counterbalanced by decreasing the risk of disease development and by using the most appropriate therapeutic weapons in the single patient. This would finally allow a much better allocation of human and economic resources.
How do you minimise the use of technologies that are ineffective or harmful?
This is precisely where HTA finds its main strength. The expert HTA panels are responsible for reviewing applications and periodically updating diagnostics lists, to account for improvements in technology and shifting disease epidemiology. In brief, the main goal of HTA is the careful evaluation of a medical or diagnostic technology for evidence of safety, efficacy, cost-effectiveness, but also considering the potential ethical, legal and social implications. The demonstration of no costeffectiveness of a given test of technology may also support laboratory professionals to eliminate tests, by presenting objective data to clinicians and to all the other stakeholders (i.e., patients and relatives).
What
is the current status of the HTA Review and its recommendations?
Several lines of evidence now demonstrate that HTA should be regarded as one element providing multiple values to innovation in laboratory medicine. Nevertheless, HTA analyses of diagnostics tests remain quite infrequent in the international literature. There is a compelling need to fill this gap and scientific societies and organisations should be proactive or promoting (and participating to) HTA in laboratory medicine.
















